1) Welcome to Part 2 of a new #accredited #tweetorial in our series of educational programs on #hypertrophic #cardiomyopathy #HCM. DOn't miss Part 1, still available for 🆓CE/#CME, at cardiometabolic-ce.com/hcm5a/.
Now you can learn more, and EARN MORE credit, by following this 🧵!
Now you can learn more, and EARN MORE credit, by following this 🧵!
2) Our expert author is again Sergio Kaiser MD PhD FACC FESC 🇧🇷🇮🇱 @pabeda1, cardiologist 🫀, Professor 🎓 of #InternalMedicine, Rio de Janeiro State University. He brings the general cardiologist's perspective to our #HCM discussions. Read and learn!
#FOAMed #CardioTwitter
#FOAMed #CardioTwitter

3) This program is supported by an unrestricted educational grant from Bristol Myers Squibb. Statement of accreditation and faculty disclosures at cardiometabolic-ce.com/disclosures/. Credit for #physicians #nursepractitioners #physicianassociates #nurses #pharmacists from @academiccme.
4a) So we promised at the end of Part 1 to talk about advances in #medical therapy for #HCM, and we alluded to the mechanism of #myosin inhibition in that regard.
The first report of a small molecule capable of preventing development of #HCM in a 🐁model dates back to 2016.
The first report of a small molecule capable of preventing development of #HCM in a 🐁model dates back to 2016.
4b) MYK-461, later known as #mavacamten, ⬇️ contractility by ⬇️#ATP activity of the cardiac #myosin heavy chain. Its early, chronic administration ⬇️suppressed development of #LVH, cardiomyocyte disarray, & myocardial fibrosis.
4c) This also attenuated hypertrophic and #profibrotic gene expression in mice harboring heterozygous human mutations in the # myosin heavy chain (🔓pubmed.ncbi.nlm.nih.gov/26912705/).
4d) From pubmed.ncbi.nlm.nih.gov/32897741/, here schematically is the mode of action of #mavacamten. 

5) The development program for #mavacamten in human beings suffering from HCM has been well described: 

6a) The dose-finding, open-label, non-randomized Ph 2 study #PIONEER_HCM enrolled 2 cohorts of #oHCM pts w/sx: 1⃣ w/10-20mg 1⃣ w/2-5 mg. There was improvement in post-exercise #LVOT gradients, exercise capacity, & symptoms.
6b) Tolerance to treatment was good, despite a more pronounced decrease in # LVEF in those treated with the higher dose.
pubmed.ncbi.nlm.nih.gov/31035291/
pubmed.ncbi.nlm.nih.gov/31035291/

7a) The dose-finding #DBRCT Ph 2 #MAVERICK_HCM in symptomatic pts w/non-obstructive #HCM(34) randomized 1:1:1 to placebo or #mavacanten 200 or 500 mg, then followed 16 weeks.
See 🔓pubmed.ncbi.nlm.nih.gov/32466879/.
See 🔓pubmed.ncbi.nlm.nih.gov/32466879/.
7b) Biomarkers of ventricular wall stress (#NT_proBNP and cardiac #troponin I) were significantly ⬇️ in both groups. Serious adverse events occurred in 10% of participants on mavacamten and in 21% participants on placebo.
(Group 1=200mg dose, Group 2=500mg dose)

(Group 1=200mg dose, Group 2=500mg dose)


8a) #Explorer_HCM, a #RDBPCT led by @cardiomet_CE faculty @IacopoOlivotto (pubmed.ncbi.nlm.nih.gov/32871100/) enrolled pts w/#oHCM + #LVOT gradient =/> 50 mm Hg + #NYHA class II–III sx assigned (1:1) to #mavacamten (w/ dose adjustments at 8 & 14 wks) or placebo for 30 weeks.
8b) #Mavacamten doses 2.5, 5, 10, or 15mg given PO to ➡️target ⬇️in #LVOT gradient < 30mm Hg + mavacamten plasma concentration 350-700ng/mL. 75% of pts taking beta-blockers & 18.7% taking #CCBs at enrollment. 

8c) Study endpoints are shown below.
👉251 patients were enrolled and randomly assigned to #mavacamten (49%) or placebo (51%).
👉Among patients on mavacamten, 37% met the primary EP vs 17% of those on placebo (difference +19·4%, 95% CI 8·7 to 30·1; p=0·0005).
👉251 patients were enrolled and randomly assigned to #mavacamten (49%) or placebo (51%).
👉Among patients on mavacamten, 37% met the primary EP vs 17% of those on placebo (difference +19·4%, 95% CI 8·7 to 30·1; p=0·0005).

8d) Pts on #mavacamten had
🫀 greater ⬇️ vs placebo in post-exercise #LVOT gradient
🫀 greater ⬆️in pVO2
🫀 improved symptom scores
Improvement by at least 1⃣NYHA class in 34% more patients in the mavacamten group.


🫀 greater ⬇️ vs placebo in post-exercise #LVOT gradient
🫀 greater ⬆️in pVO2
🫀 improved symptom scores
Improvement by at least 1⃣NYHA class in 34% more patients in the mavacamten group.



8e) Safety and tolerability of #mavacamten were similar to placebo.
There were also significant drops in NT-proBNP and #cTNI among patients assigned to mavacamten as compared to those on placebo.
There were also significant drops in NT-proBNP and #cTNI among patients assigned to mavacamten as compared to those on placebo.

8f) #symptom relief? per Kansas City Cardiomyopathy Score #KCCS
👉 At 30wks, change in #KCCQ_OS (overall score) sig greater w/#mavacamtenvs placebo, & % pts w/ a very large change = 36% vs 15% ➡️#NNT=5.
👉 Gains returned to baseline after tx interrupted.
pubmed.ncbi.nlm.nih.gov/34004177/
👉 At 30wks, change in #KCCQ_OS (overall score) sig greater w/#mavacamtenvs placebo, & % pts w/ a very large change = 36% vs 15% ➡️#NNT=5.
👉 Gains returned to baseline after tx interrupted.
pubmed.ncbi.nlm.nih.gov/34004177/
8g) Scores grouped into clinically worse (change in score, baseline to wk 30, –5 points or less), no sig change (> −5 to < 5 pts), small but clinically imp't improve (5 to < 10 pts), mod-large clinical improve (10 to < 20 pts), & large to very large clinical improve (20+ pts). 

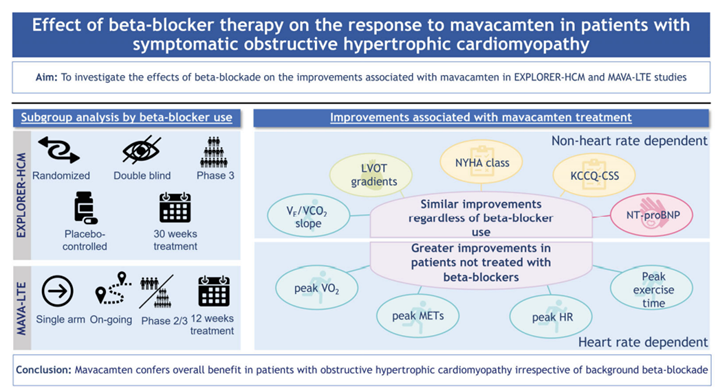
9a) We talked in Part 1 about "classic" therapy for #HCM, eg beta-blockers. An interesting analysis of pts from #EXPLORER_HCM & #MAVA_LTE (ongoing Ph 2/3 extension study of long-term #mavacamten (252 weeks) after completion of EXPLORER or MAVERICK.
9b) 👁️at interaction of #BB & #mavacamten on exercise parameters (🔓pubmed.ncbi.nlm.nih.gov/36404399/).
🫀 224 pts, 169 taking, 55 not taking #BBs
👉data suggest pts intolerant to BBs can derive similar benefit from #macavamten
🫀 224 pts, 169 taking, 55 not taking #BBs
👉data suggest pts intolerant to BBs can derive similar benefit from #macavamten
10a) At #ACC22, a combined analysis of #MAVA_LTE & #EXPLORER_LTEdemonstrated benefits from the parent studies were maintained at a 62-week median follow-up period. #LVEF ⬇️7-9%, as expected.
10b) Always worth emphasizing that modest ⬇️in #LVEF derive from the mode of action of #myosin inhibitors
👉usu not clinically sig
👉reversible when drug d/c
👉drug can be restarted at lower doses once EF returns to previous values

👉usu not clinically sig
👉reversible when drug d/c
👉drug can be restarted at lower doses once EF returns to previous values


11) In April 2022 @US_FDA approved #mavacantem for the treatment of adults with #NYHA class II-III #oHCM to improve functional capacity and symptoms. Approval included a #REMS monitoring requirement.
12a) Given what you know about the impact of #mavacamten on #LVEF, what do you suppose is the primary means of patient monitoring in the #REMS?
a. Periodic #NT_pro_BNPassay
b. Periodic #CMR
c. Periodic #echocardiogram
d. Periodic #troponin I assay
a. Periodic #NT_pro_BNPassay
b. Periodic #CMR
c. Periodic #echocardiogram
d. Periodic #troponin I assay
12b) Mark your best answer and RETURN TOMORROW for the correct response, more education, an intro to @aficamten, and a link to your 🆓CE/#CME!
👍 @alexariasmx20 @KemalogluOz @Sarah_Moharem @DrCCaroli @vitorborin_ @omendiz @VerwerftJan @bcaramelli @rmourilhe @EzequielZaidel
👍 @alexariasmx20 @KemalogluOz @Sarah_Moharem @DrCCaroli @vitorborin_ @omendiz @VerwerftJan @bcaramelli @rmourilhe @EzequielZaidel
13) Welcome back! We are talking all about #HCM from the general #cardiology perspective with expert author @pabeda1 🇧🇷🇮🇱 and you are earning LOTS of 🆓CE/#CME by following this 🧵
#CardioTwitter #MedTwitter #MedEd @MedTweetorials @PreMedTweets @4hcm
#CardioTwitter #MedTwitter #MedEd @MedTweetorials @PreMedTweets @4hcm
14a) Yesterday's quiz? The correct answer is C: #echocardiograms. A Risk Evaluation and Mitigation Strategy #REMS is a drug safety program that @US_FDA can require for certain medicines to ensure they are used safely.
14b) For #mavacamten, this includes focused patient education & #echocardiogramsmay also be needed:
🫀 4 weeks after a change in the dose
🫀 after a break in tx (as determined by the HCP)
🫀 4 weeks and 12 weeks after starting certain 💊that are known to affect mavacamten
🫀 4 weeks after a change in the dose
🫀 after a break in tx (as determined by the HCP)
🫀 4 weeks and 12 weeks after starting certain 💊that are known to affect mavacamten
15) Now, as promised, on to more #mavacemten data and a look at the emerging #myosin inhibitor, #aficamten.
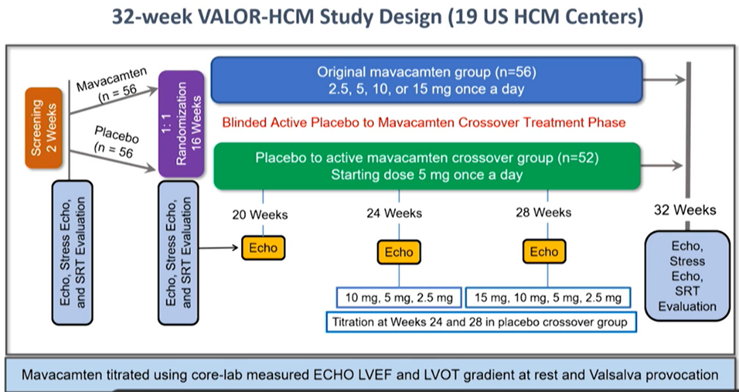
16a) So, more data: #VALOR_HCM, a #RDBPC trial, aimed to assess #mavacamten in pts w/ #oHCM who were referred for or actively considering septal reduction tx #SRT. Included:
🫀 at least 18
🫀 LV wall thickness ≥ 15 mm (13 mm if familial)
🫀 #NYHA class 3 or higher (2 if syncope)
🫀 at least 18
🫀 LV wall thickness ≥ 15 mm (13 mm if familial)
🫀 #NYHA class 3 or higher (2 if syncope)
16b (cont)
🫀 resting or provoked #LVOT gradient ≥ 50 mmHg
🫀 LVEF ≥ 60%
In this trial, use of disopyramide at baseline allowed. Pts randomized to an escalating dose of #mavacamtenor placebo, followed 16 weeks:
🔓 pubmed.ncbi.nlm.nih.gov/35798455/
🫀 resting or provoked #LVOT gradient ≥ 50 mmHg
🫀 LVEF ≥ 60%
In this trial, use of disopyramide at baseline allowed. Pts randomized to an escalating dose of #mavacamtenor placebo, followed 16 weeks:
🔓 pubmed.ncbi.nlm.nih.gov/35798455/

16c) Results: After 16 weeks only 18% of #mavacamten pts remained eligible for, or underwent #SRT, vs 77% in the placebo group. 🔑efficacy & safety endpoints shown here: 



16d) No patient had a dangerous drop in #LVEF. Two patients on mavacamten experienced a >50% ⤵️in EF, but returned to baseline values after transient drug discontinuation.
17a) Then 52 placebo-tx pts were switched to open-label #mavacamten & compared to 52 pts who stayed on study med, then followed 32 wks.
pubmed.ncbi.nlm.nih.gov/36335531/
pubmed.ncbi.nlm.nih.gov/36335531/
17b) At 32 weeks, 10.7% in original #mavacamten group & 13.5% in the placebo cross-over group met #SRT guideline criteria or elected to undergo SRT. The mava group showed sustained ⬇️in resting #LVOT gradient and Valsalva LVOT gradient 

17c) Similar gradient reductions shown in the cross-over group after 16 weeks of #mavacamten.
🫀 Also, improvement ≥1 #NYHA class was observed in 90.6% from the original mavacamten group and in 70% from the cross-over group after 16 weeks.

🫀 Also, improvement ≥1 #NYHA class was observed in 90.6% from the original mavacamten group and in 70% from the cross-over group after 16 weeks.


17d) In #VALOR_HCM, #mavacamten also ➡️significant improvements in the degree of mitral regurgitation #MR and in systolic motion of the anterior mitral valve leaflet #SAM: 



18a) And now let's shift gears⚙️
🫀 #Aficamten is 2nd in class selective inhibitor of cardiac #myosin that acts by binding directly at a distinct allosteric binding site ➡️⬇️number of actin–myosin cross-bridges causing #HCM hypercontractility.
🫀 #Aficamten is 2nd in class selective inhibitor of cardiac #myosin that acts by binding directly at a distinct allosteric binding site ➡️⬇️number of actin–myosin cross-bridges causing #HCM hypercontractility.

18b) #REDWOOD_HCMwas Ph 2 dose-finding #RPCT of #aficamten.
👉enrolled 2 sequential cohorts of pts w/ symptomatic #oHCM
👉1 tx'd w/ 3 escalating doses of 5 to 15 mg vs placebo, 1 tx'd w/ 3 escalating doses of 10 to 30 mg vs placebo
🔓 pubmed.ncbi.nlm.nih.gov/36599608/
👉enrolled 2 sequential cohorts of pts w/ symptomatic #oHCM
👉1 tx'd w/ 3 escalating doses of 5 to 15 mg vs placebo, 1 tx'd w/ 3 escalating doses of 10 to 30 mg vs placebo
🔓 pubmed.ncbi.nlm.nih.gov/36599608/
18c) Primary objective: determine safety & tolerability of various doses # aficamten.
🔑secondary EPs:
🫀 change from baseline resting & Valsalva #LVOT gradients over 10 weeks
🫀 % w/ complete hemodynamic response
🫀 change from baseline #LVEF, #biomarkers, #NYHA functional class



🔑secondary EPs:
🫀 change from baseline resting & Valsalva #LVOT gradients over 10 weeks
🫀 % w/ complete hemodynamic response
🫀 change from baseline #LVEF, #biomarkers, #NYHA functional class

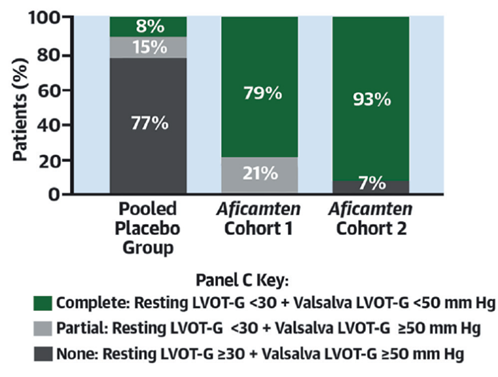


18d) In both cohorts, #aficamten induced a robust reversal of LVOT gradient, while no patient experienced a reduction of #LVEF that prompted drug discontinuation. 

19a) Given results of #REDWOOD_HCM, #aficamten was tested for 12 weeks in a 4th cohort of pts w/ non-obstructive #HCM.
🆕Results were presented at ACC 23 by @cardiomet_CE expert faculty @MasriAhmadMD:
🆕Results were presented at ACC 23 by @cardiomet_CE expert faculty @MasriAhmadMD:

19b) 85% of pts achieved max 15 mg dose & no pt had #LVEF drop <50%. In this trial, 54% of patients improved by at least one functional class. There were also significant drops in NT-proBNP and hs-TNI over time. Trial medication was withheld after 10 weeks 



20a) #FOREST_HCM is an ongoing open-label extension study of patients with #oHCM or non-oHCM who completed the parent short-term studies. Pts were titrated to a max dose of 20 mg #aficamten if tolerated. 

20b) Among these pts, 42% were eligible for #SRT at baseline and 22% were taking #disopyramide. Main results are shown here: 





21) So, let's start to wrap this up. We've been on quite a ride. Again, if you missed last week's part 1 of this #tweetorial, you can check it out & still earn CE/#CME at cardiometabolic-ce.com/hcm5a/.
22a) Where do cardiac #myosin inhibitors fit into management of #oHCM? A suggested decision flow for use of #macavamten from
📖 Ommen SR #ACC22 oral presentation
📖 pubmed.ncbi.nlm.nih.gov/32871100/
📖 🔓 pubmed.ncbi.nlm.nih.gov/35798455/
📖 Lakdawala 2023
📖#mavacamten PI: accessdata.fda.gov/drugsatfda_doc…

📖 Ommen SR #ACC22 oral presentation
📖 pubmed.ncbi.nlm.nih.gov/32871100/
📖 🔓 pubmed.ncbi.nlm.nih.gov/35798455/
📖 Lakdawala 2023
📖#mavacamten PI: accessdata.fda.gov/drugsatfda_doc…


23a) So as we summarize, let's see what you learned. Which of the following re #HCM is FALSE?
a. genetic testing is not required for diagnosis.
b. echo and #CMR are complementary imaging methods
c. mavacamten is approved for #oHCM
d. mavacamten is approved for non-oHCM
a. genetic testing is not required for diagnosis.
b. echo and #CMR are complementary imaging methods
c. mavacamten is approved for #oHCM
d. mavacamten is approved for non-oHCM
23b) It's d. #Mavacamten is a #myosin inhibitor approved to treat obstructive #HCM in symptomatic patients. Non-#oHCM is currently managed with #beta_blockers, #CCBs, #ACEIs, and sometimes diuretics.
24a) On to take-🏡messages:
1⃣ Hypertrophic cardiomyopathy #HCM is now compatible w/ longevity & good quality of life, thanks to advances in assessment & tx of its phenotypic expressions. But, periodic reassessment will be a companion over the life course of a patient with HCM.
1⃣ Hypertrophic cardiomyopathy #HCM is now compatible w/ longevity & good quality of life, thanks to advances in assessment & tx of its phenotypic expressions. But, periodic reassessment will be a companion over the life course of a patient with HCM.
24b)
2⃣The diagnosis of #HCM is built on clinical grounds (history, signs and symptoms) coupled to cardiac imaging. Genetic testing is not essential for diagnosis and does not predict prognosis.
2⃣The diagnosis of #HCM is built on clinical grounds (history, signs and symptoms) coupled to cardiac imaging. Genetic testing is not essential for diagnosis and does not predict prognosis.
24c)
3⃣Genetic testing is recommended for famílial screening, though probands harboring a sarcomeric mutation may never develop the clinical and morphological spectrum of HCM. Indeed, phenotypic features of HCM cannot be fully explained by genetics.
3⃣Genetic testing is recommended for famílial screening, though probands harboring a sarcomeric mutation may never develop the clinical and morphological spectrum of HCM. Indeed, phenotypic features of HCM cannot be fully explained by genetics.
24d) Still, #genetic screening 🧬is key because even w/out symptoms, offspring members who inherit a #sarcomeric mutation must be followed up over time.
24d)
4⃣Echocardiography and cardiac MR are complementary methods for assessing hypertrophy patterns, cardiac systolic and diastolic function, and features of prognostic importance, like apical aneurisms, myocardial fibrosis and massive hypertrophy.
4⃣Echocardiography and cardiac MR are complementary methods for assessing hypertrophy patterns, cardiac systolic and diastolic function, and features of prognostic importance, like apical aneurisms, myocardial fibrosis and massive hypertrophy.
24e)
5⃣ #ICD implant in patients at risk for sudden death #SCD is associated with long-term survival and good quality of life
5⃣ #ICD implant in patients at risk for sudden death #SCD is associated with long-term survival and good quality of life
24f)
6⃣Septal #myectomy or alcohol ablation are established procedures for preventing #heartfailurein selected cases of #oHCM, but should be performed in centers with high volume of procedures.
6⃣Septal #myectomy or alcohol ablation are established procedures for preventing #heartfailurein selected cases of #oHCM, but should be performed in centers with high volume of procedures.
24g)
7⃣Cardiac #myosin inhibitors represent the first tx approach targeting modulation of the contractile abnormality. Efficacy & safety have been documented in dedicated studies but long-term confirmation of persistent benefits are still pending.
7⃣Cardiac #myosin inhibitors represent the first tx approach targeting modulation of the contractile abnormality. Efficacy & safety have been documented in dedicated studies but long-term confirmation of persistent benefits are still pending.
24h)
8⃣ #Macavamtenis already approved in the U.S. for the treatment of adults with symptomatic #NYHA class II-III obstructive #HCM to improve functional capacity and symptoms. #Aficamten is under study.
8⃣ #Macavamtenis already approved in the U.S. for the treatment of adults with symptomatic #NYHA class II-III obstructive #HCM to improve functional capacity and symptoms. #Aficamten is under study.
24i)
9⃣ During tx with cardiac myosin inhibitors, a 5% ⤵️ in #LVEF is expected & thus echocardiographic monitoring is necessary. Do not initiate if LVEF <55%, & interrupt use if LVEF <50%. After restoration of LVEF, the drug can be restarted at a lower dose.
9⃣ During tx with cardiac myosin inhibitors, a 5% ⤵️ in #LVEF is expected & thus echocardiographic monitoring is necessary. Do not initiate if LVEF <55%, & interrupt use if LVEF <50%. After restoration of LVEF, the drug can be restarted at a lower dose.
25) And you just earned ANOTHER 0.75h 🆓CE/#CME. Claim it with a few clicks of the 🖱️at cardiometabolic-ce.com/hcm5b/. I am @pabeda1 and I thank you for joining me on this journey!
• • •
Missing some Tweet in this thread? You can try to
force a refresh

 Read on Twitter
Read on Twitter














