Why does normal saline (NS) contain 154 mEq of sodium per liter if a normal serum sodium is ~140 mEq/L?
This has bugged me for YEARS.
I’ll do my best to explain a potential reason below. As always, I’d love to hear from others, either confirming or refuting what follows…
Next: two questions.
First, which [Na+] does your lab report on a chemistry panel (e.g, BMP, chem7, etc.)?
Second, which is the following is the physiologically important [Na+]?
Answers:
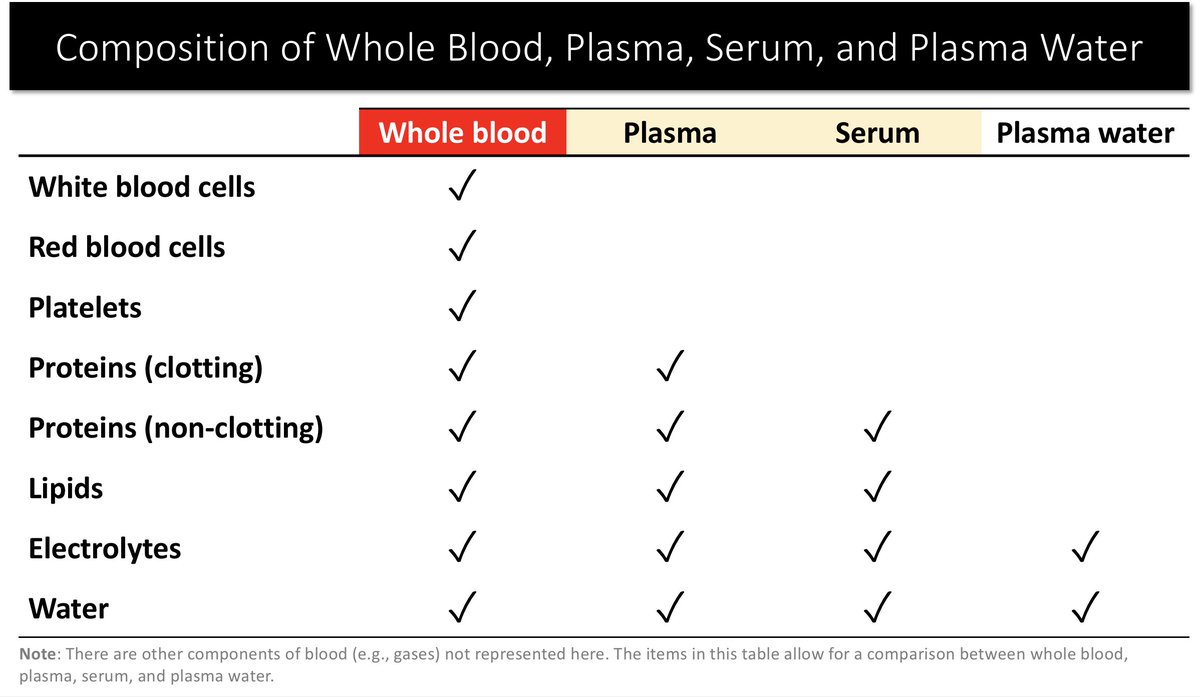
1. The [Na+] reported in most labs is the plasma [Na+].
2. Sodium exists in the aqueous (water) part of plasma. Therefore, the plasma water [Na+] is the physiologically important one.
Again:
*Labs report: plasma [Na+]
*Physiologically important: plasma water [Na+]
It may not seem obvious, but it’s so important:
Plasma ≠ Plasma Water (see #2 above).
This leads to another question: What percent of plasma is typically composed of water?
Based on the above, what is the plasma water [Na+]? Let’s assume a plasma sodium of 143 mEq/L and plasma that is 93% water.
Almost there.
Last question: In normal saline, what is the NaCl dissolved in?
Normal saline is NaCl dissolved in water. For our purposes, it’s basically plasma water. It should come as no surprise that NS matches the body fluid to which it is most similar (i.e., plasma water).
Both are water + electrolytes.
Make sense?
To summarize:
Normal serum [Na+]: ~143
Normal serum water [Na+]: ~154
Normal saline [Na+]: 154
NS matches serum water (not serum!) given they’re both water + electrolytes.
Much of the above is used to understand pseudohyponatremia. Would anyone be interested in a thread explaining this?