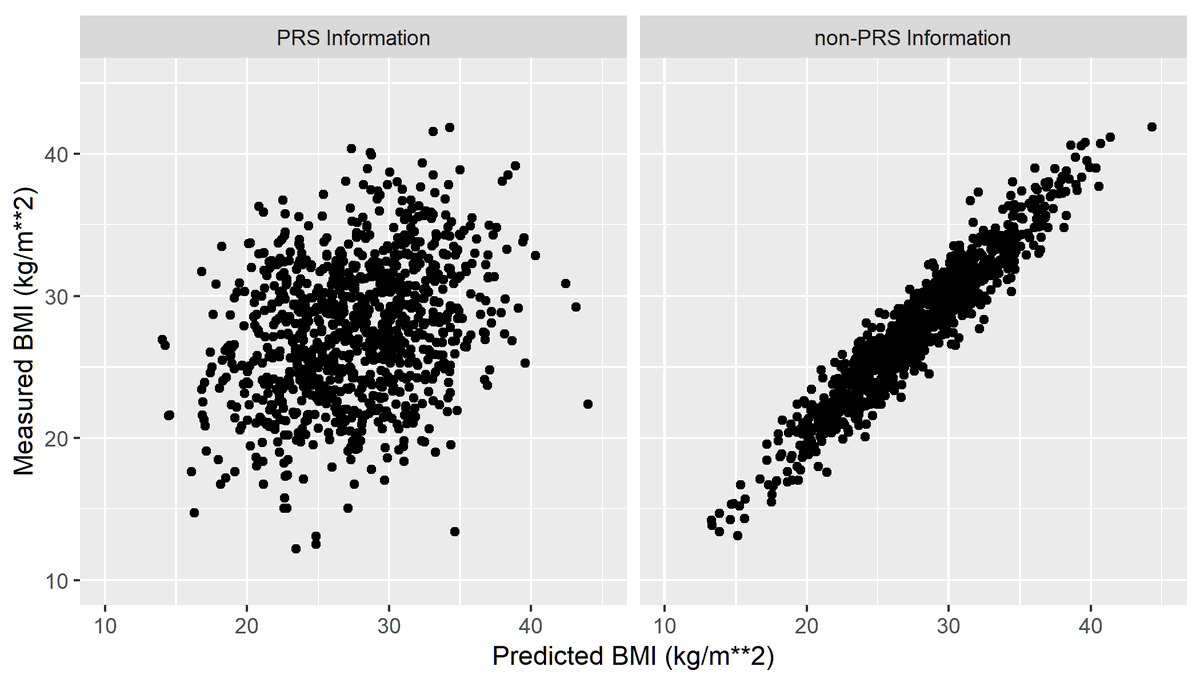
imaging.onlinejacc.org/content/12/7_P…
This is a non-inferiority trial but I can't make the math add up.
Follow along...
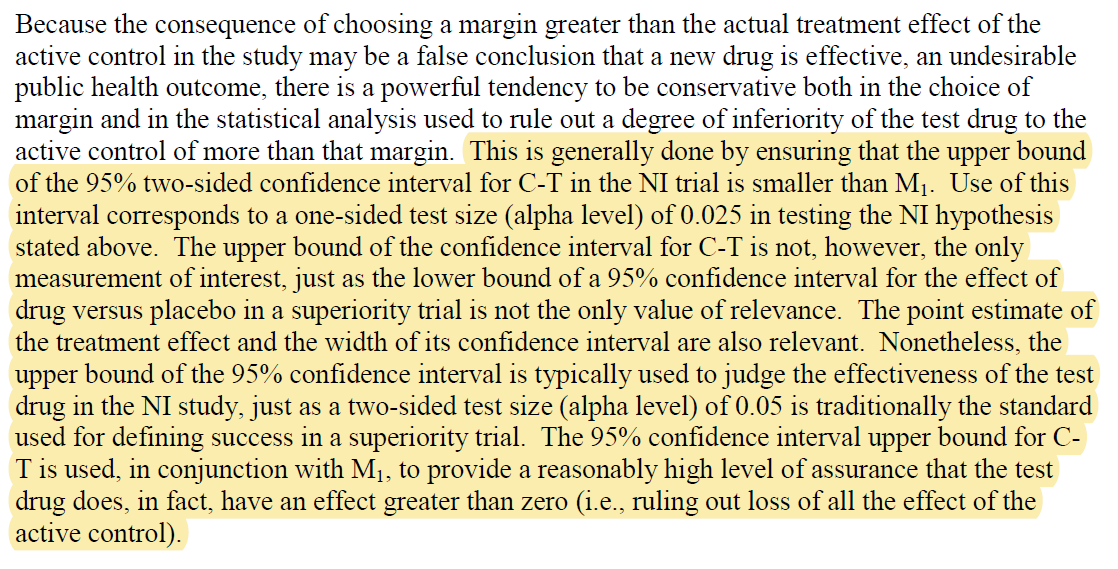
Usually this is upper bound for MACE in cardiology trials showing new tx doesn't result in significantly more MACE than old tx.
Some trials have tested upper bounds of non-inferiority of as high 1.5 or even higher maybe.
This means CCTA patients could experience 33% more MACE events than ICA and still be non-inferior.
Why set them this high? Because if you don't the sample sizes get VERY large very quickly.
Interesting. Is 1.47>1.33?
Simple: they didn't ask whether the 95% CI crossed 1.33, they asked whether the 90% CI crossed 1.33
sciencedirect.com/science/articl…
fda.gov/media/78504/do…
Let's do the math, shall we?
Likewise the lower 95% confidence interval for is given by taking the estimate for beta and subtracting 1.96 times it standard error.
Dividing that by 2.93 gives us 0.204.
So we now know beta is -0.010 and has a standard error of 0.204
-0.010 + 1.645 * 0.204 = 0.326
e^0.326 = 1.39
That's pretty close to 1.33 but still crosses it.
* Very high non-inferiority margin
* 95% CI crosses the margin
* 90% CI crosses the margin
But somehow it met its non-inferiority endpoint.
I think that it is important.
Do you?
What's above shows the trial did NOT make its primary endpoint, despite the claim that it did
They did not demonstrate non-inferiority. They happened to have HR of 1.0 but the trial is compatible with a markedly worse event rate.