There are four serotypes of the dengue virus.
Infection with one serotype does not fully protect one from contracting a different serotype.
The mechanism for why is fascinating.
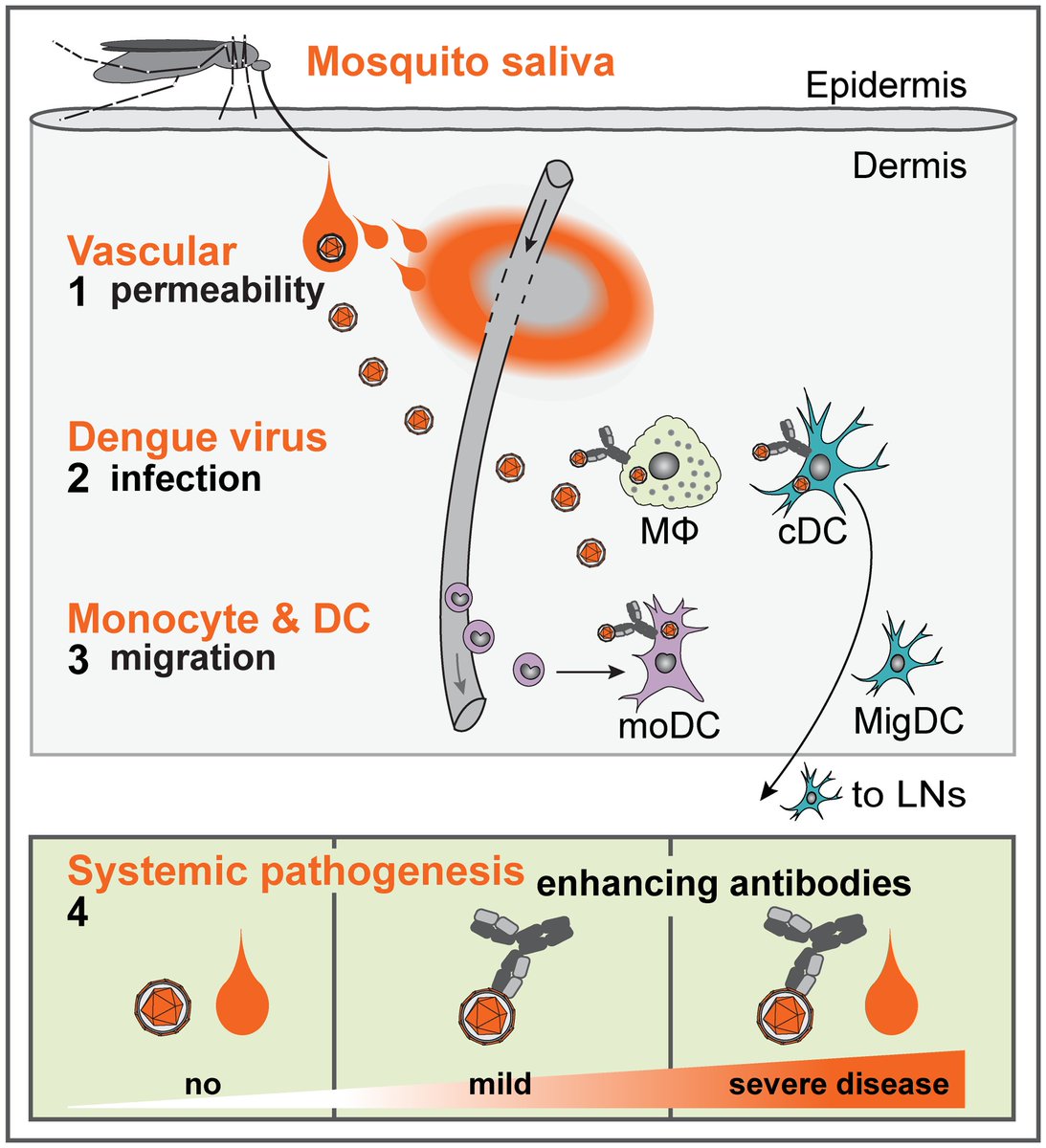
It is then transported to nearby lymph nodes, ultimately creating a neutralizing antibody-mediated response.
...dengue has a trick up its sleeve, though.
What is it?
Cytokine storm ensues.
Vascular endothelial dysfunction occurs, leading to a prothrombotic state.
Local mast cell granule release leads to further capillary leak.
Eventually, hemorrhagic shock ensues.
Make an isolated strong neutralizing antibody? You rapidly clear the virus.
Make little to no antibody? You tend not to manifest severe disease or hemorrhagic complications.
Similar to Dengue, there are 5 non-SARS coronavirus serotypes, and we are quick to forget they compose the vast majority of coronavirus infections.
They tend to cause mild URI symptoms and readily circulate through the human population.
Per a Finnish study:
<2 yo = no measurable coronavirus antibodies
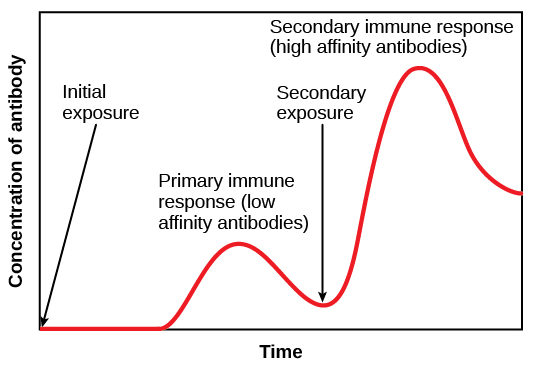
2-6 yo = a rapid increase in antibody prevalence
Antibody levels continue to increase until 10-14 years, likely the result of cross-serotype boosting.
Like Dengue, coronavirus antibodies are known to cross-react between serotypes.
Antibody dependent enhancement has been shown to occur in human coronaviruses and is not a novel concept:
ncbi.nlm.nih.gov/pubmed/32092539
virologyj.biomedcentral.com/articles/10.11…
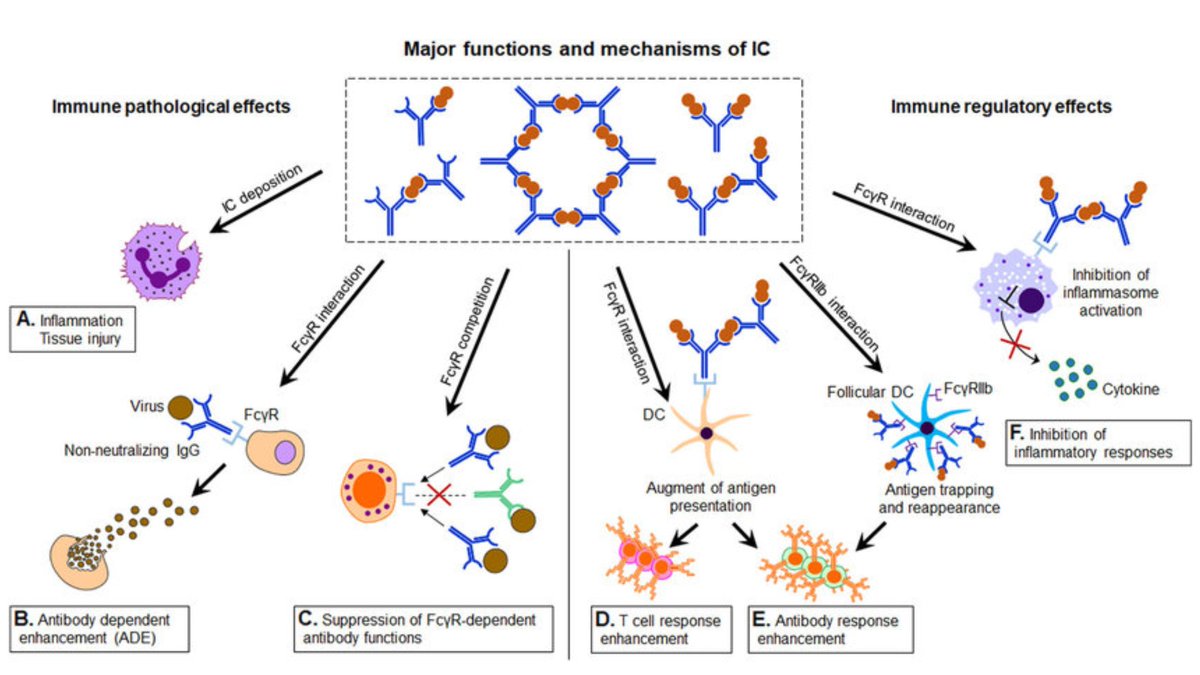
(For those that don't remember: Type III = antigen/antibody complex formation --> capillary interstitial deposition --> immune activation --> endothelial damage [see below])
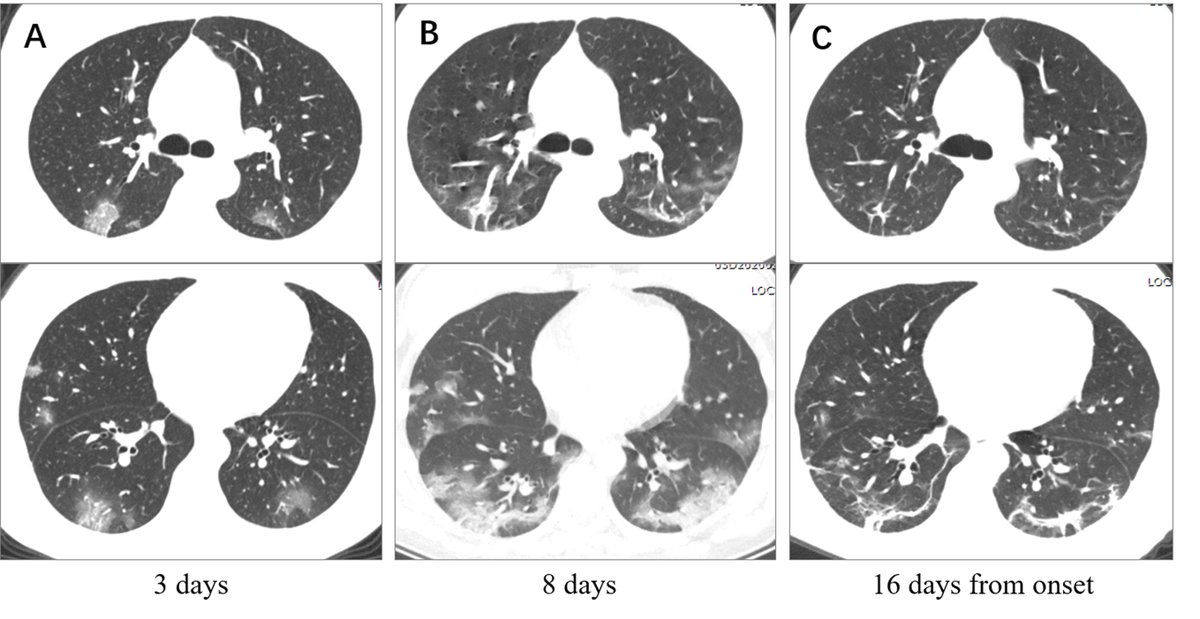
Patients are poor to oxygenate but have adequate lung compliance, especially early on.
This fits with interstitial edema > intra-alveolar edema and also explains why high PEEP is often needed in these patients.
1. Non-SARS coronaviruses are ubiquitous
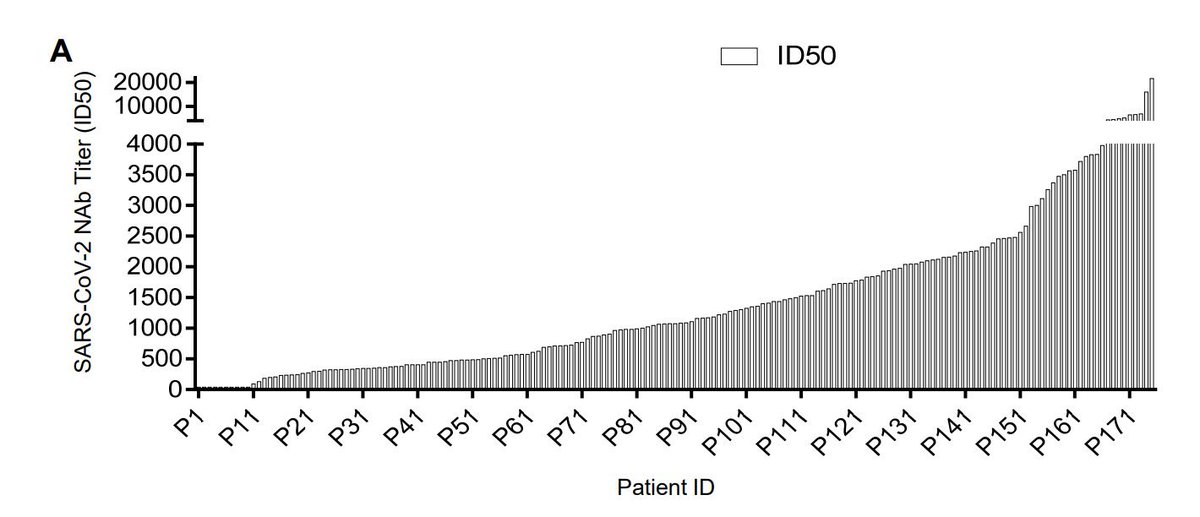
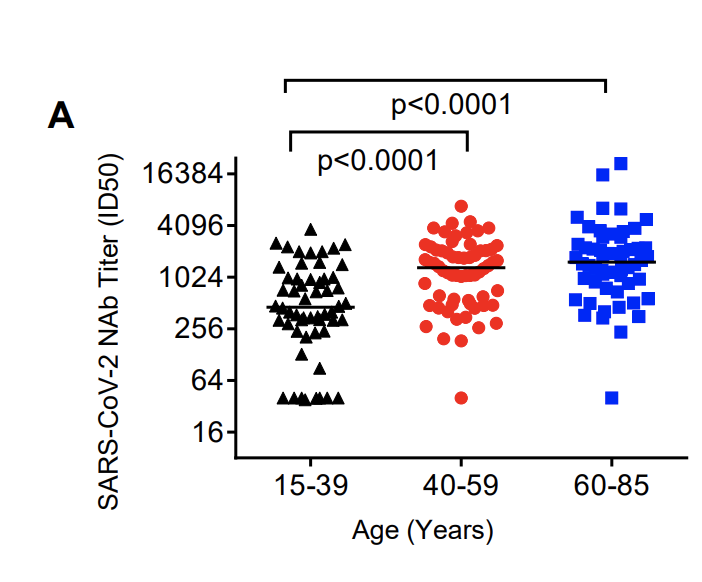
2. Antibody levels parallel age
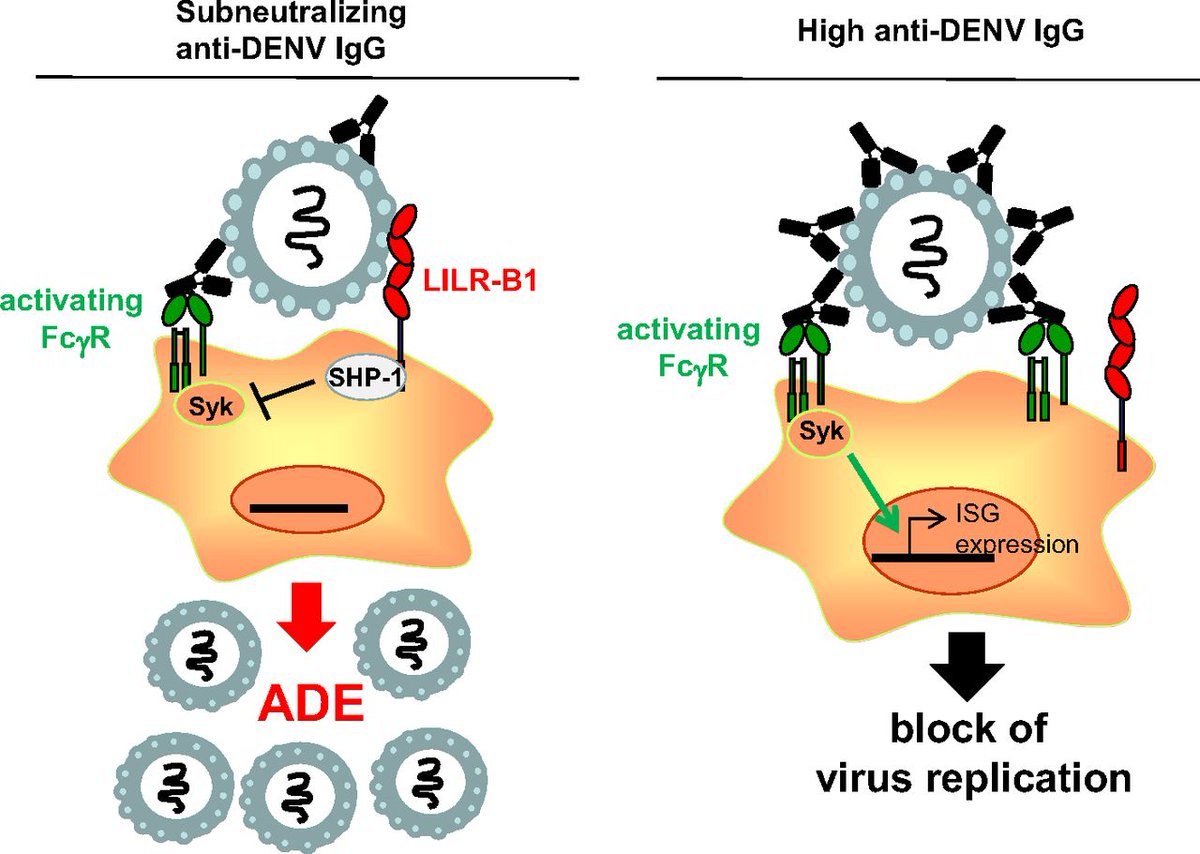
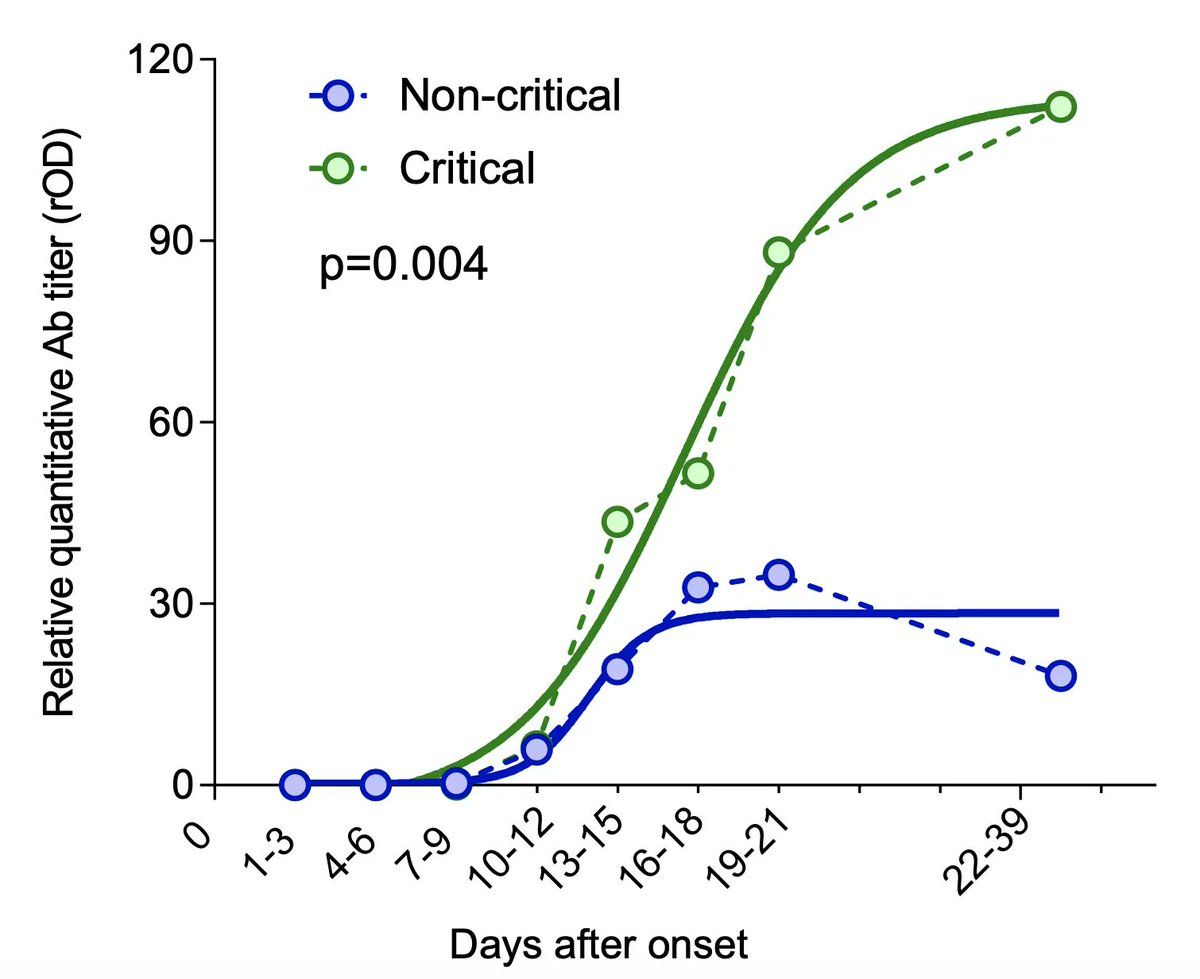
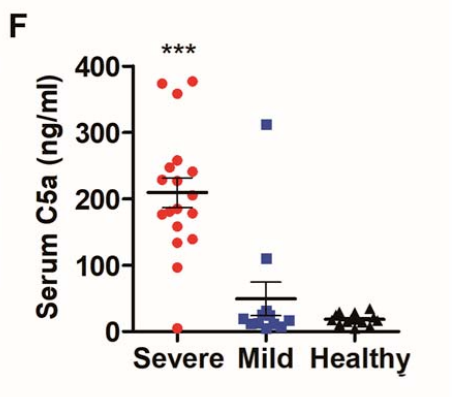
3. Sub-neutralizing antibodies are key, as they enhance viremia and disease severity
4. Ab/ag complex deposition may drive severe disease phenotype via type III / IV hypersensitivity rxns
Part 2: