
THREAD
There’s much debate around the UK's recommended use of the AZ vaccine with a two-dose schedule and flexible timing of second dose. Some thoughts on the AZ recommendation (not Pfizer) based on available data with refs to some excellent threads. 1/
There’s much debate around the UK's recommended use of the AZ vaccine with a two-dose schedule and flexible timing of second dose. Some thoughts on the AZ recommendation (not Pfizer) based on available data with refs to some excellent threads. 1/
https://twitter.com/HelenBranswell/status/1344306446910058496?s=20
UK’s MHRA and JCVI are highly-experienced in vaccine assessments and recommendations, and they've surely weighed the benefits & risks of this recommendation carefully. That said, it would be good to see all the data underpinning their recommendation. 2/
https://twitter.com/notdred/status/1344248984571936768?s=20
In general, vaccines should be taken on a schedule tested in an efficacy trial. But it wasn’t possible to conduct the typical dose and schedule optimization prior to these Ph3 trials, and those trials provided valuable data to inform these recommendations. 3/
The UK recommends a two-dose schedule, with the second dose between 4-12 weeks. This *is not* a single dose schedule. Given the data provided, and in the setting of limited supply, overstretched hospitals, and emergence of a more transmissible variant, this seems justifiable. 4/
The UK has important data on the AZ Vx that wasn’t available for Pfizer & Moderna at FDA's VRBPAC, including:
* single-dose efficacy through 4+ months; and
* single-dose immunogenicity (12+ weeks). 5/
* single-dose efficacy through 4+ months; and
* single-dose immunogenicity (12+ weeks). 5/
https://twitter.com/dgurdasani1/status/1344727697524740101?s=20
The data shows the AZ vaccine maintains efficacy in the setting of a delayed second dose, although it’s not clear how fast this wanes over time. Delay of the second dose provides a better booster effect as measured by antibody levels, which is seen with other vaccines. 6/ 



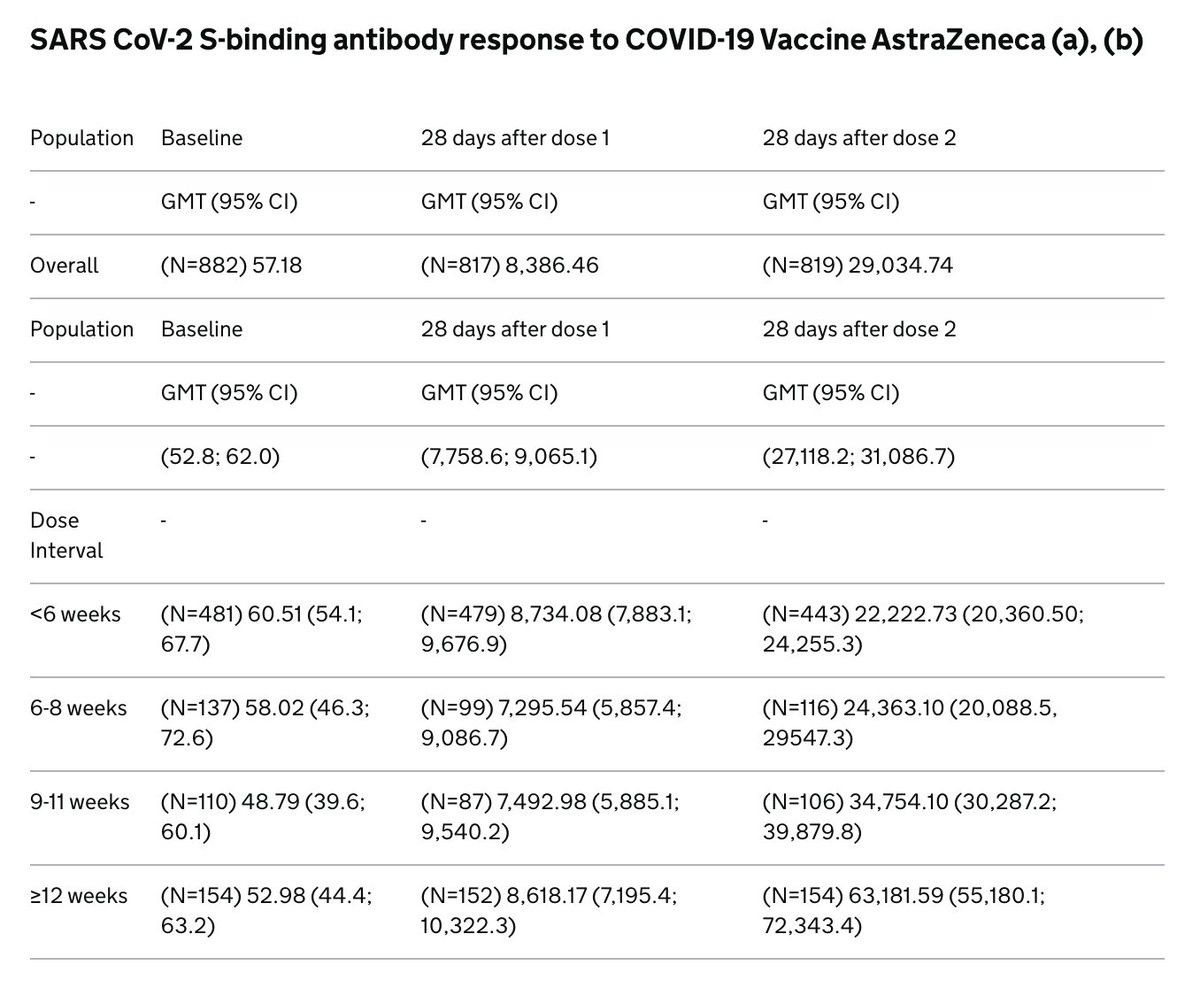
The second dose is important because it can drive a more robust and higher quality antibody response through a process called affinity maturation. It's well-understood that longer intervals can provide a better boost. 7/
https://twitter.com/sandyddouglas/status/1344949258483621888?s=20
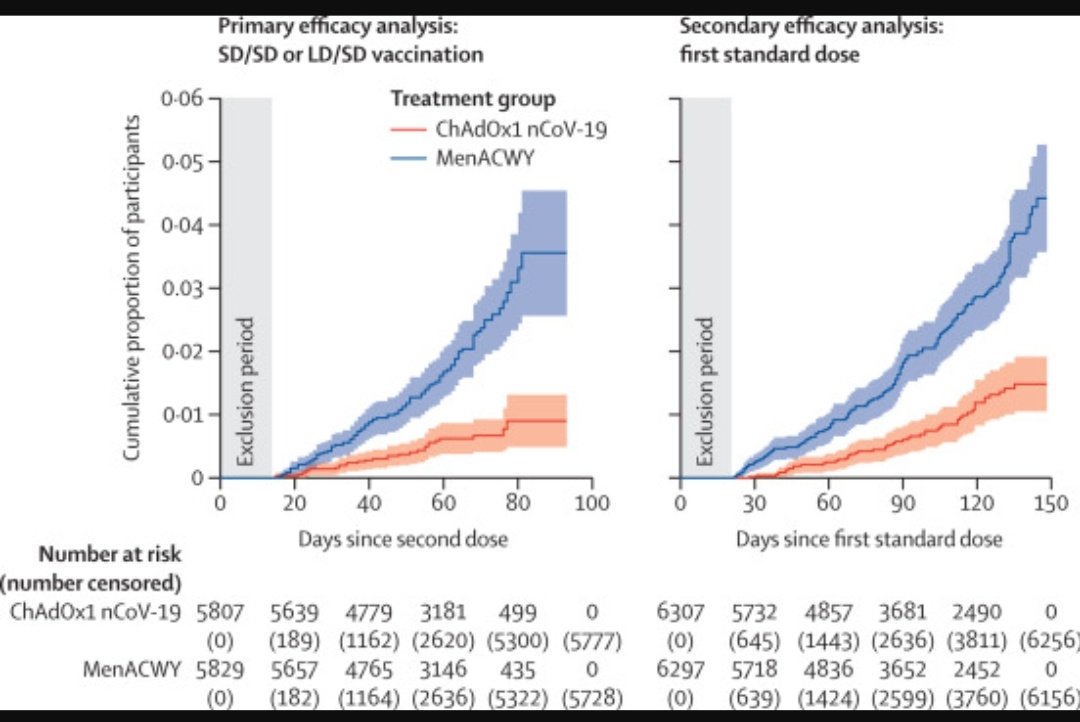
In my view, the immediate priority is prevention of severe disease that drives deaths and the strain on health systems. Efficacy against severe disease is likely better than overall vaccine efficacy. Data after the first dose is promising although the numbers are small. 8/ 
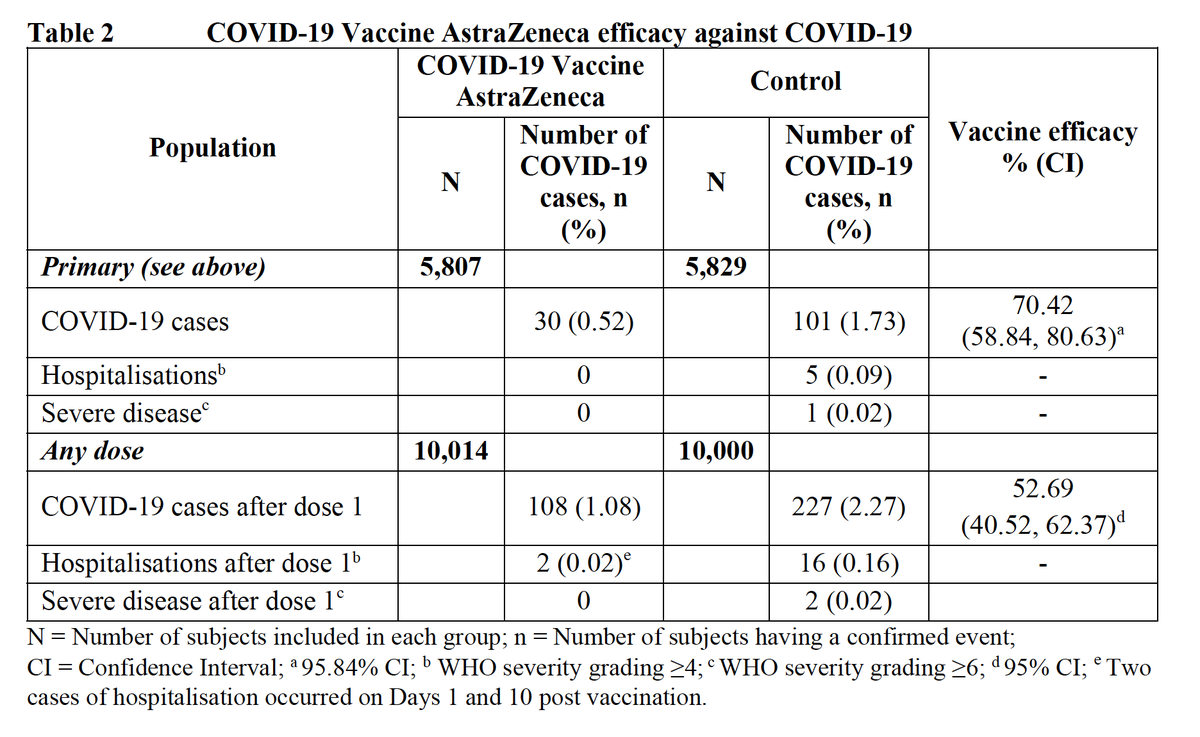
There’s nothing magic about the (short) 28d interval between doses, which was presumably chosen to ensure rapid onset of protection in Ph3. Vaccine efficacy won't disappear overnight with a delayed 2nd dose - it will wane over time, if at all. Ph3 data supports that concept. 9/
Yes there are risks of waning immunity, non-compliance with the second dose, confusion among the public, etc. Many are implementation considerations that can be addressed with planning and strong communication. 10/
It will be critically important to collect efficacy data around this “flexible second dose” schedule, esp in older adults & against severe disease, to inform licensure and recommendations in countries around the world that are counting on the introduction of this vaccine. 11/
Yes the AZ/Oxford studies had a number of issues, but we must remember that this highly-complex development program was executed in less than a year. And that Pfizer & Moderna's Ph3 execution & vaccine efficacy created very high expectations for all subsequent programs. 12/
The bottom line is that every country needs to make difficult policy decisions with the data they have, not what they'd like to have. There's no perfect answer here, but now that a decision has been taken, the UK can focus on maximizing the benefit and mitigating any risks. 13/
I didn't address question of adjusting the mRNA vaccine schedule, mainly due to lack of data, and was glad to see @VirusesImmunity posted her expert thoughts on the topic. 14/
https://twitter.com/VirusesImmunity/status/1345086669607890945?s=20
• • •
Missing some Tweet in this thread? You can try to
force a refresh



