
CEO, Aerium Therapeutics; CEPI & IAVI Boards. Fmr Head Takeda Vaccines; Fmr White House & Gates Foundation; Pulm & Crit Care; @ucsf @umich; views mine.
4 subscribers
How to get URL link on X (Twitter) App


 While P2G3 is similar to bebtelovimab, there are important differences. Virus escape studies show that P2G3 neutralizes BA.4 viruses that "escape" bebtelovimab, but bebtelovimab does not neutralize P2G3 escapees. CryoEM helps explain the different behavior. 2/ @Turelli_EPFL
While P2G3 is similar to bebtelovimab, there are important differences. Virus escape studies show that P2G3 neutralizes BA.4 viruses that "escape" bebtelovimab, but bebtelovimab does not neutralize P2G3 escapees. CryoEM helps explain the different behavior. 2/ @Turelli_EPFL 

https://twitter.com/gabegutierrez/status/1430515300001255424An exponential fight requires exponential weapons that control the transmission driving exponential growth: vaccines for all eligible, universal masking, TTI, ventilation, etc. This makes it possible for treatment capacity to keep up with demand. 2/
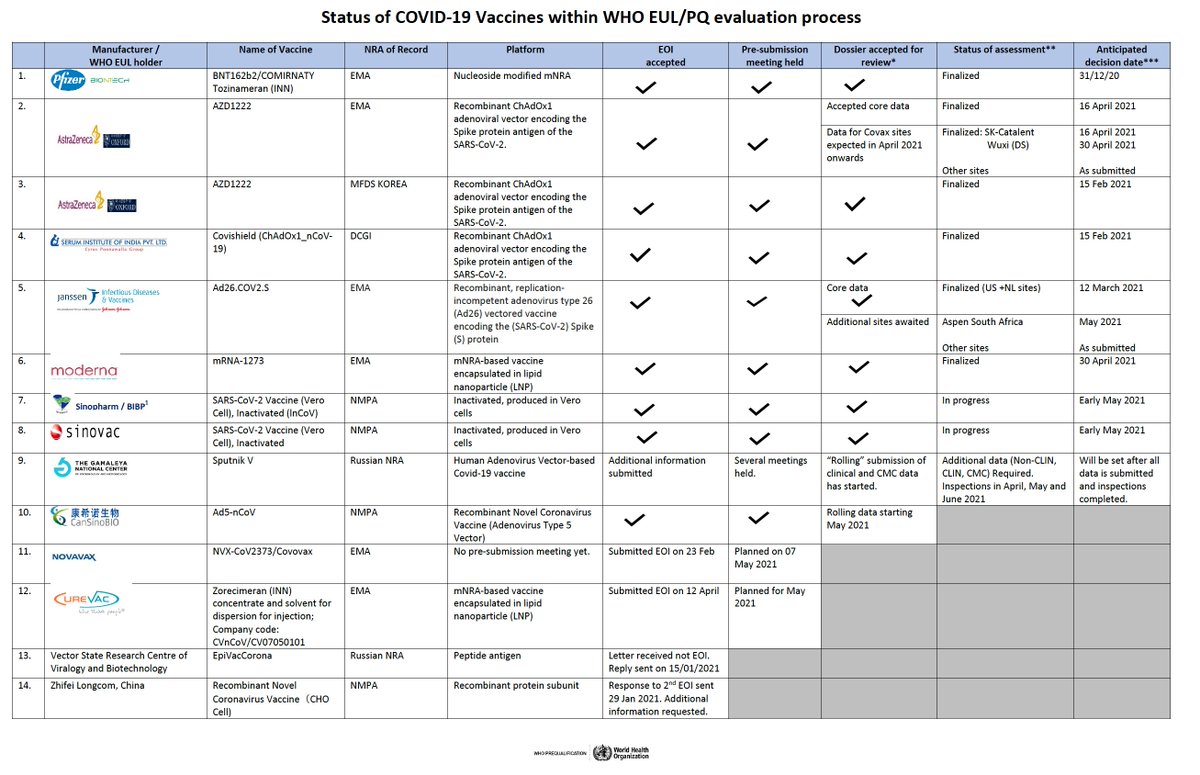
https://twitter.com/HelenBranswell/status/1390274848392368133Scaling up vaccine manufacturing is *far* more complex than HIV medicines or other drugs. And the safety and benefit/risk calculation is completely different because vaccines are given to healthy people including children. 2/
https://twitter.com/rvenkayya/status/1356286204745015296?s=20



https://twitter.com/rvenkayya/status/1367544912217075716?s=20Direct protection from vaccines (protection of the vaccinated person) goes a long way toward limiting illness and death, but indirect protection massively amplifies the impact. Here’s a good explainer from @ZoeMcLaren. 2/
https://twitter.com/ZoeMcLaren/status/1373722257957195777?s=20
https://twitter.com/EricTopol/status/1367487223092760576?s=20This thread describes a world in which vaccines reduce transmission. Early evidence is promising, although more data is needed to understand magnitude of reduction and differences between vaccines. 2/
https://twitter.com/rvenkayya/status/1356286201309900800?s=20
https://twitter.com/PeterHotez/status/1358752684841525250?s=20
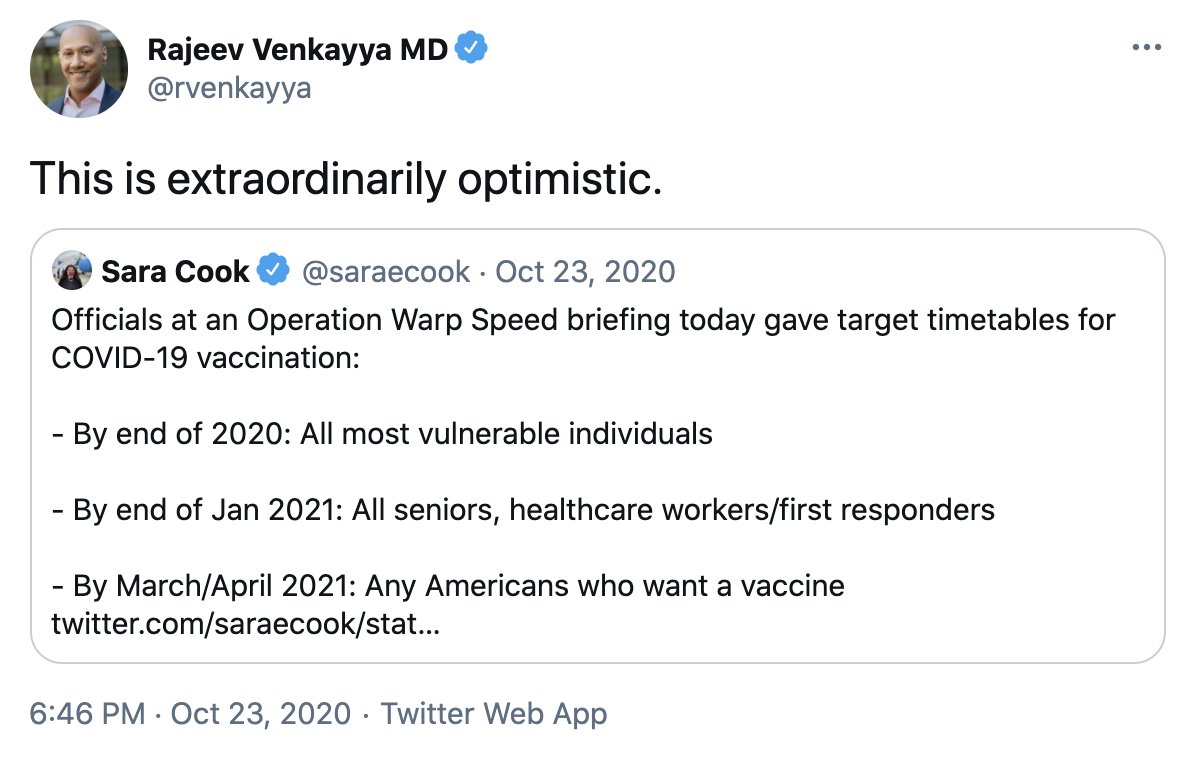
https://twitter.com/rvenkayya/status/1320022543076630529?s=20
 Much of the risk is in "scaling up" production to produce large volumes of vaccine in a facility, and “scaling out” to manufacturing partners to expand capacity. This thread is about vaccine manufacturing and the challenges we’ll continue to face. 2/
Much of the risk is in "scaling up" production to produce large volumes of vaccine in a facility, and “scaling out” to manufacturing partners to expand capacity. This thread is about vaccine manufacturing and the challenges we’ll continue to face. 2/ https://twitter.com/rvenkayya/status/1346815419810799617?s=20
https://twitter.com/rvenkayya/status/1343621750492377089?s=20Multiple lines of evidence strongly suggest mutations in B.1.351 confer some escape from natural and vaccine immunity.
https://twitter.com/K_G_Andersen/status/1355005699999158276?s=20


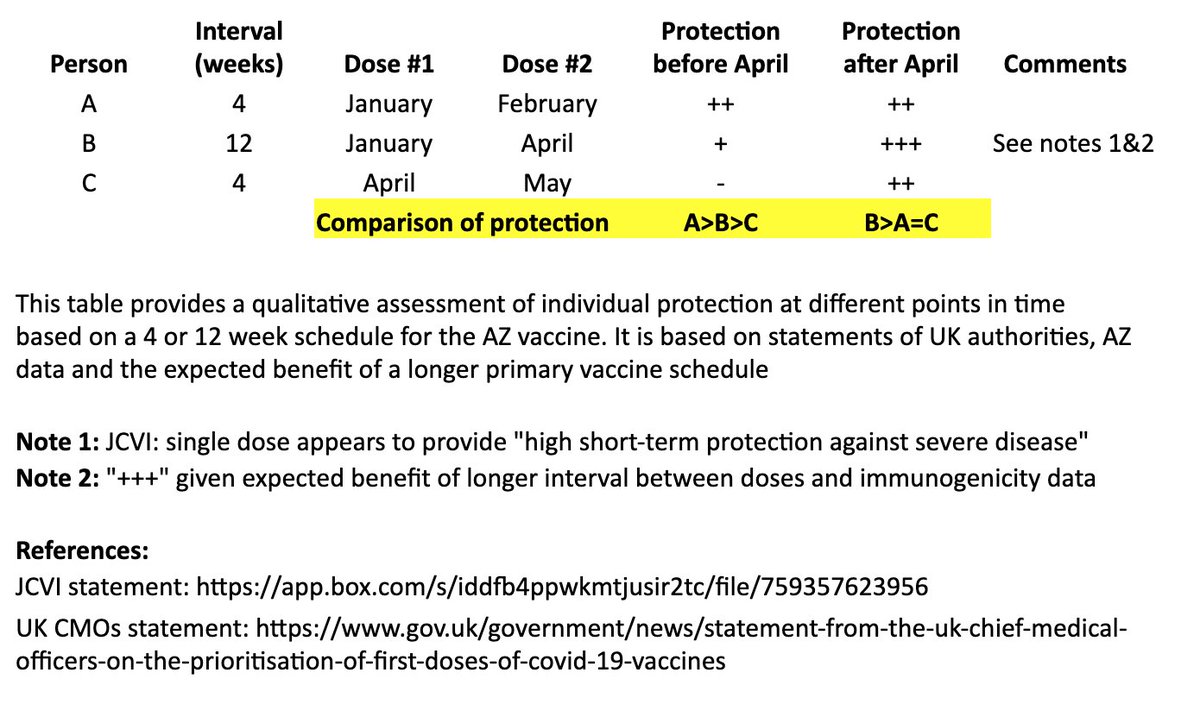
https://twitter.com/Bob_Wachter/status/1345741327330263042?s=20
 I based this on recent statements from the UK chief medical officers, JCVI, and what we know from prior vaccine development. 2/
I based this on recent statements from the UK chief medical officers, JCVI, and what we know from prior vaccine development. 2/
https://twitter.com/HelenBranswell/status/1344306446910058496?s=20UK’s MHRA and JCVI are highly-experienced in vaccine assessments and recommendations, and they've surely weighed the benefits & risks of this recommendation carefully. That said, it would be good to see all the data underpinning their recommendation. 2/
https://twitter.com/notdred/status/1344248984571936768?s=20
https://twitter.com/kakape/status/1342063459350020097The first indication of whether vaccine protection is affected will come from the lab: neutralization studies assessing whether vaccine-induced antibodies are as effective at neutralizing the new variant as earlier strains of SARS-CoV-2. Similar activity will be reassuring. 2/
https://twitter.com/bhrenton/status/1335306082693083137?s=20

https://twitter.com/notdred/status/1333555525880049664It is not a simple undertaking to establish a human challenge model, particularly with a novel virus for which the pathophysiology is not well-understood and where a targeted therapeutic is not available for what could be a lethal disease. 2/

https://twitter.com/CNNSotu/status/1330521936221392902?s=20@bnallamo @US_FDA An EUA (Emergency Use Authorization) for a vaccine is not the same as a therapeutic, given that the vaccine is being given to subgroups of people who are “healthy” and may or may not be exposed to the virus. The bar for safety & efficacy data is therefore higher than for Rx. 2/
https://twitter.com/rvenkayya/status/1325850822677303297?s=20First let’s break this down into three questions:
https://twitter.com/ashishkjha/status/1325770292199907330First, it shows vaccines *can* prevent COVID illness in humans, and it validates the spike protein target. We didn't know these things before today, and it's good news for all #COVID19 vaccines in development. 1/
https://twitter.com/saraecook/status/1319739200502194179To be clear, it's not impossible, and OWS surely has assumptions to support this. And yes we must be ambitious.