
THREAD
I created a simple table to illustrate the individual impact of the "flexible second dose timing" now recommended in the UK.
Coincidentally, @bob_wachter & @ashishkjha just tackled the US policy question in this important piece. 1/

I created a simple table to illustrate the individual impact of the "flexible second dose timing" now recommended in the UK.
Coincidentally, @bob_wachter & @ashishkjha just tackled the US policy question in this important piece. 1/
https://twitter.com/Bob_Wachter/status/1345741327330263042?s=20
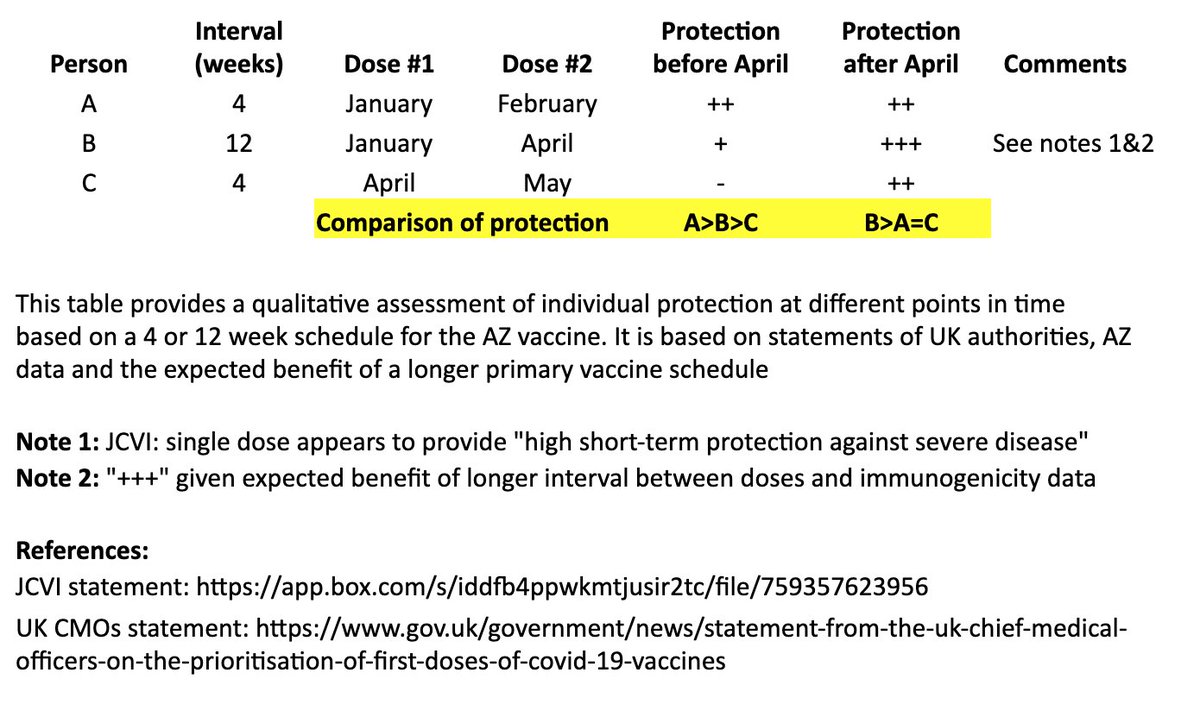
I based this on recent statements from the UK chief medical officers, JCVI, and what we know from prior vaccine development. 2/
JCVI: app.box.com/s/iddfb4ppwkmt…
UK Chief Medical Officer (CMO) statement: gov.uk/government/new…
CMO letter to the profession: gov.uk/government/pub…
JCVI: app.box.com/s/iddfb4ppwkmt…
UK Chief Medical Officer (CMO) statement: gov.uk/government/new…
CMO letter to the profession: gov.uk/government/pub…
This table and thread focuses on the AZ vaccine, where more data on a delayed second dose is available than with the Pfizer vaccine. It is not intended to address questions about single-dose regimens or mix & match approaches. 3/ 
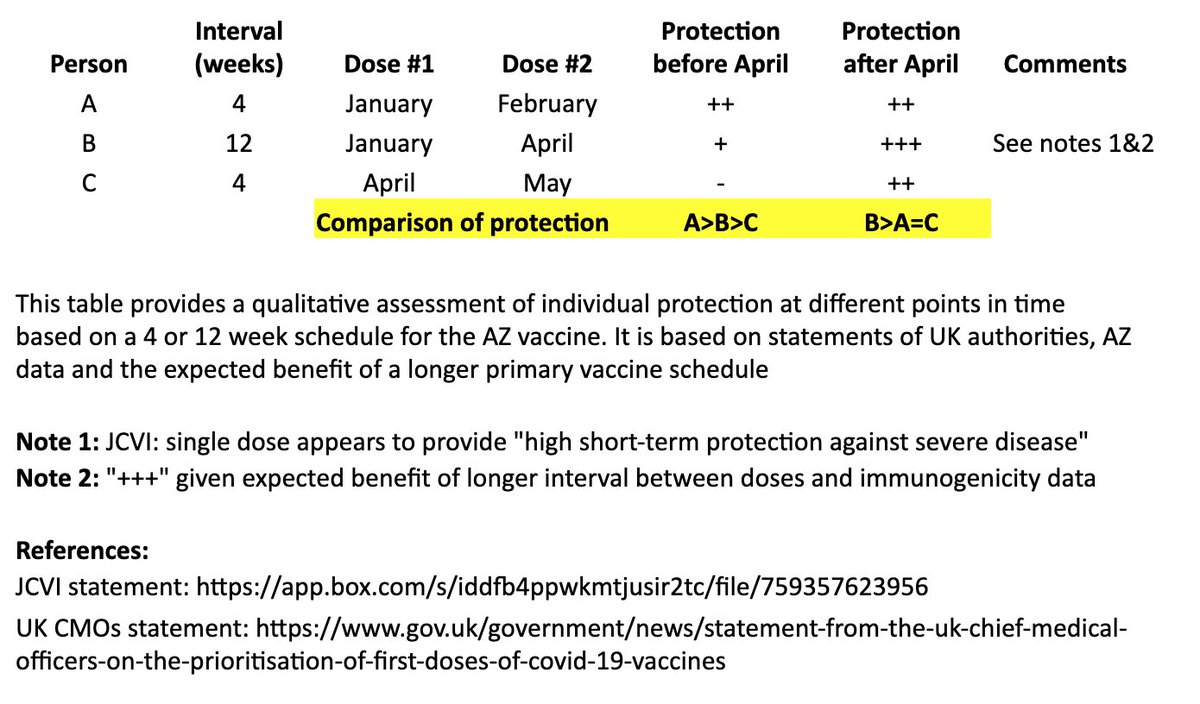
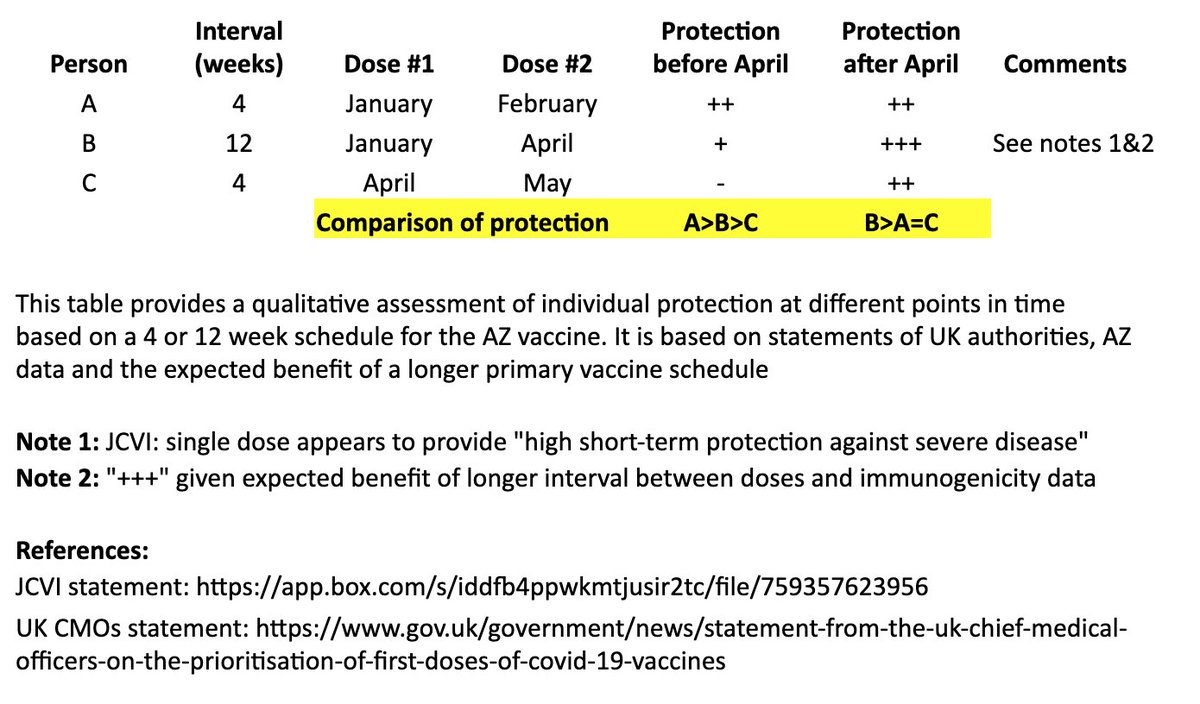
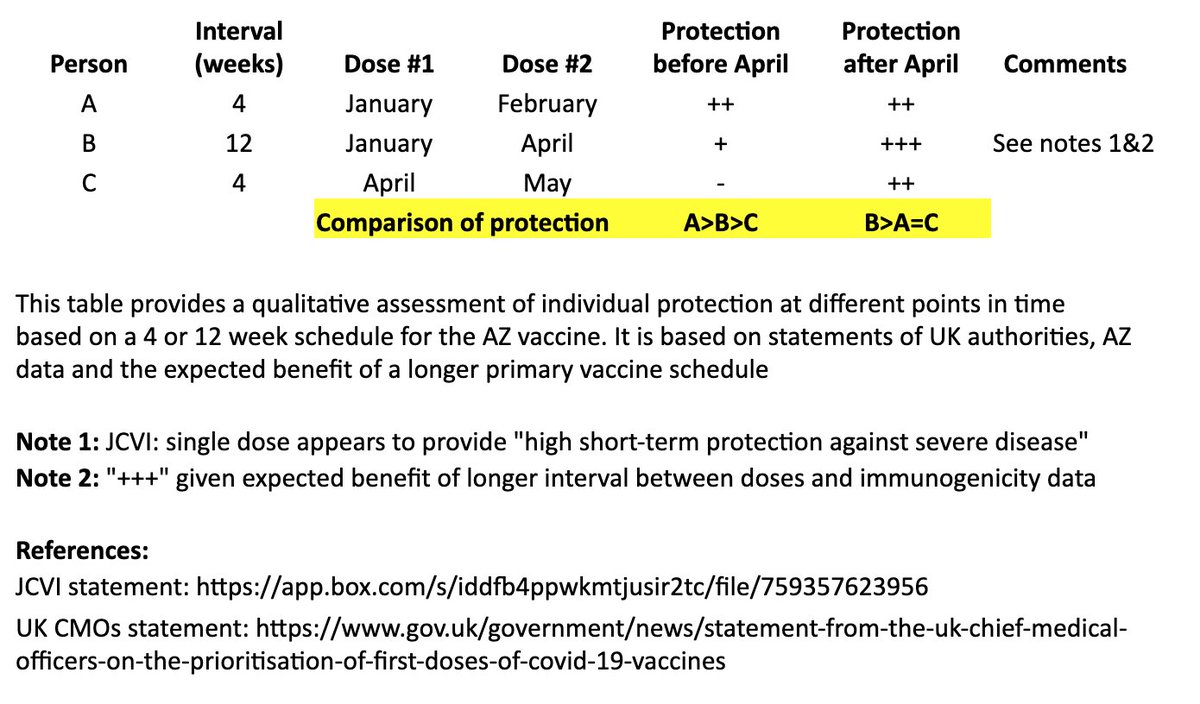
In the table, persons “A” and “B” both receive their first dose in January. “A” receives their second dose in February (4 weeks later), and “B” receives their second dose in April (12 weeks later). “C” receives their first dose in April and second dose in May (4 weeks later). 4/ 
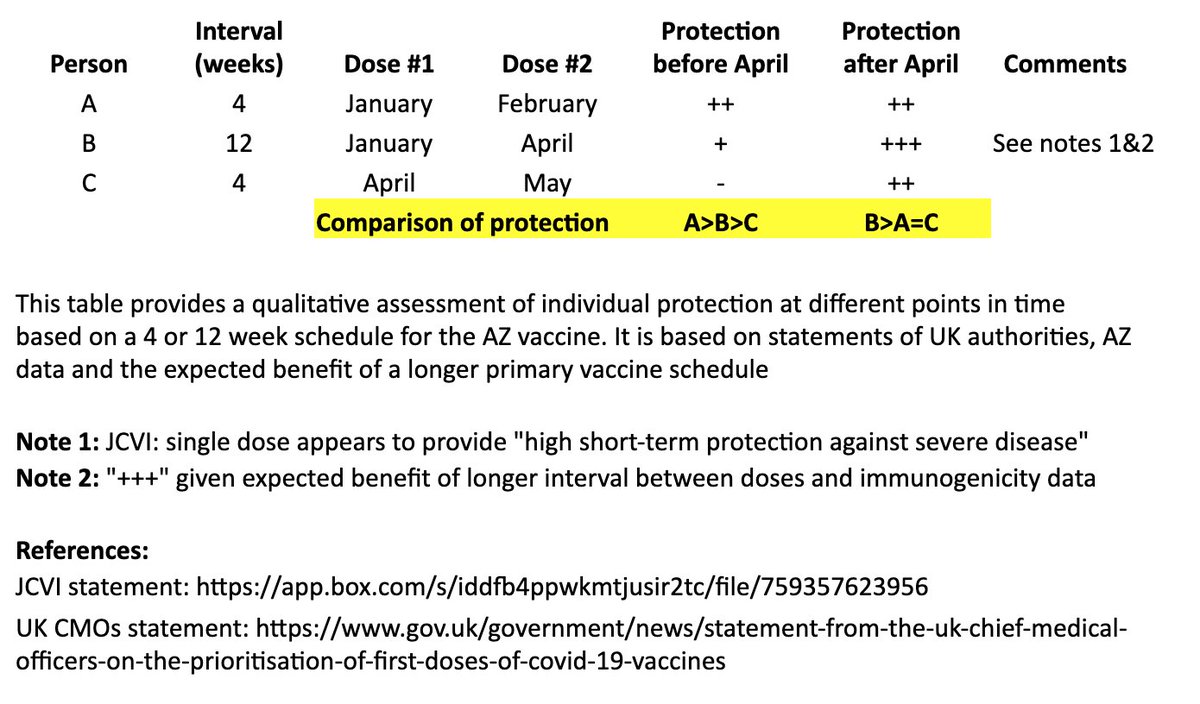
I made a qualitative comparison the potential efficacy during the two months between “A” and “B’s” second dose, as well as the potential longer-term efficacy after “B” receives their second dose. 5/ 
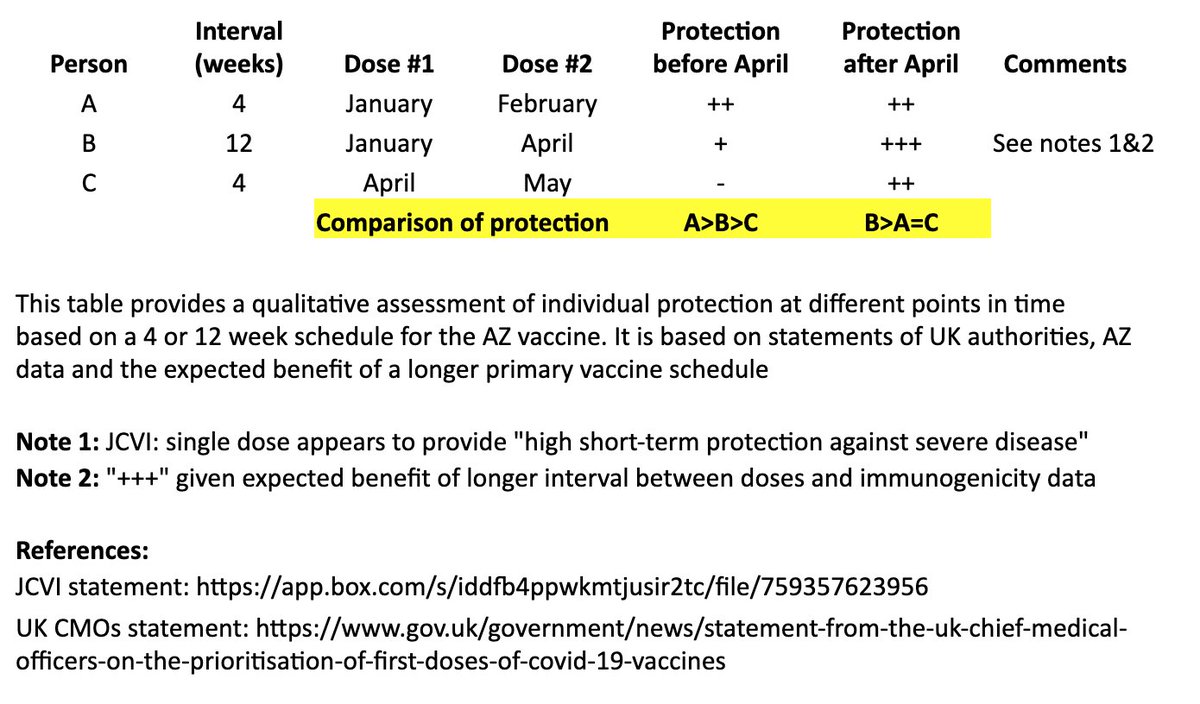
For the two-month period between the timing of “A” and “B’s” second dose, the level of protection for “A” may be greater than “B,” as “A” has received their second dose, although JCVI suggests that most of this early protection is provided by the first dose. 6/ 
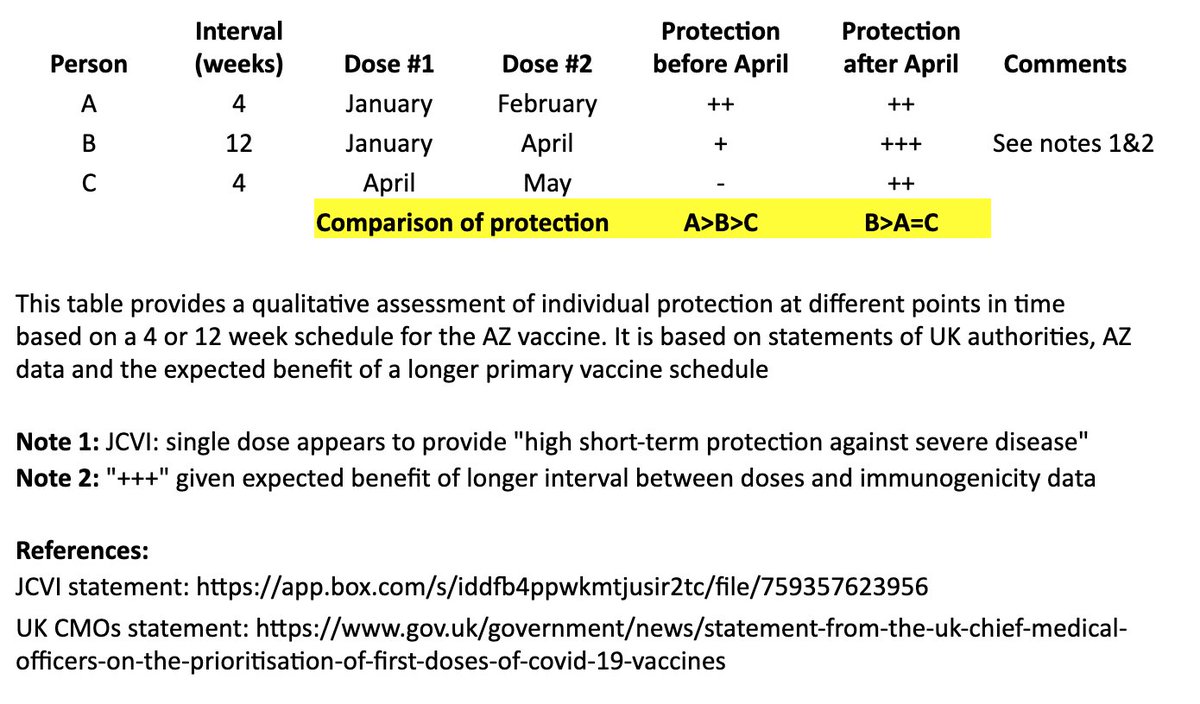
JCVI says first-dose protection against hospitalization is high, so for that two month interval, I’ve assumed “B” is expected to be protected against the more severe forms of COVID-19 (“+”). 7/ 
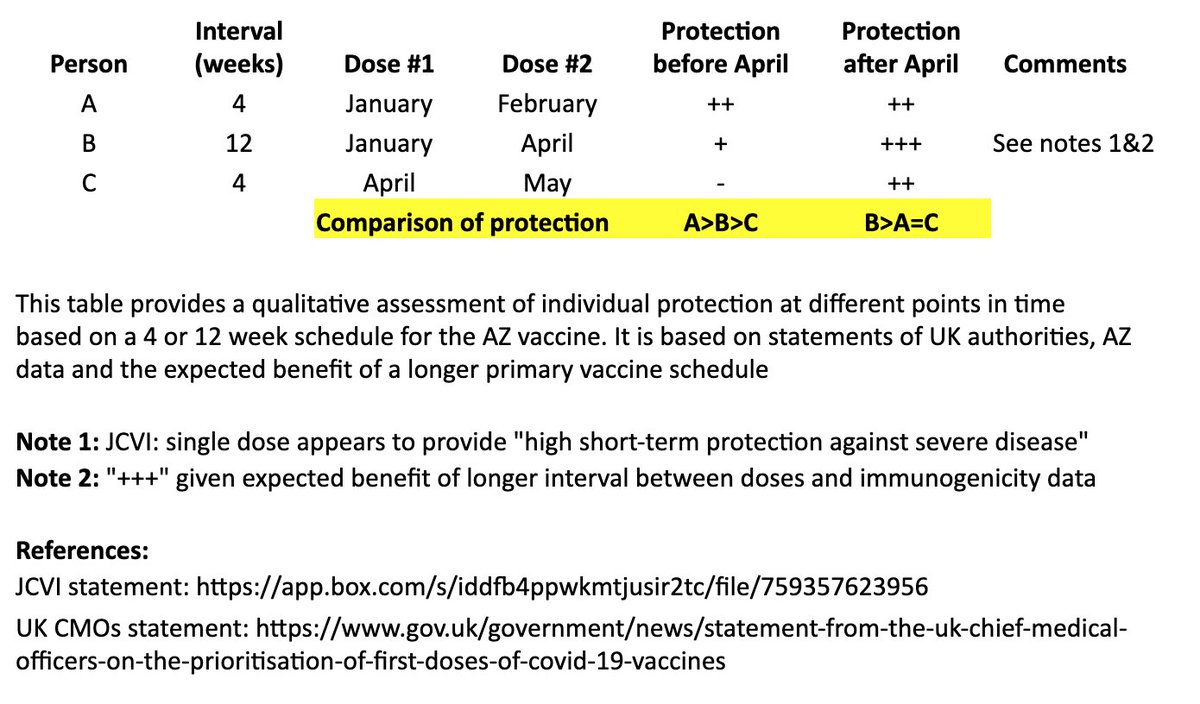
After “B’s” second dose in April, we expect a more robust, higher quality and longer-lasting immune response than “A,” as is often seen with longer intervals in a primary vaccination series and supported by AZ’s data immunogenicity data. 8/

https://twitter.com/sandyddouglas/status/1344949258483621888?s=20
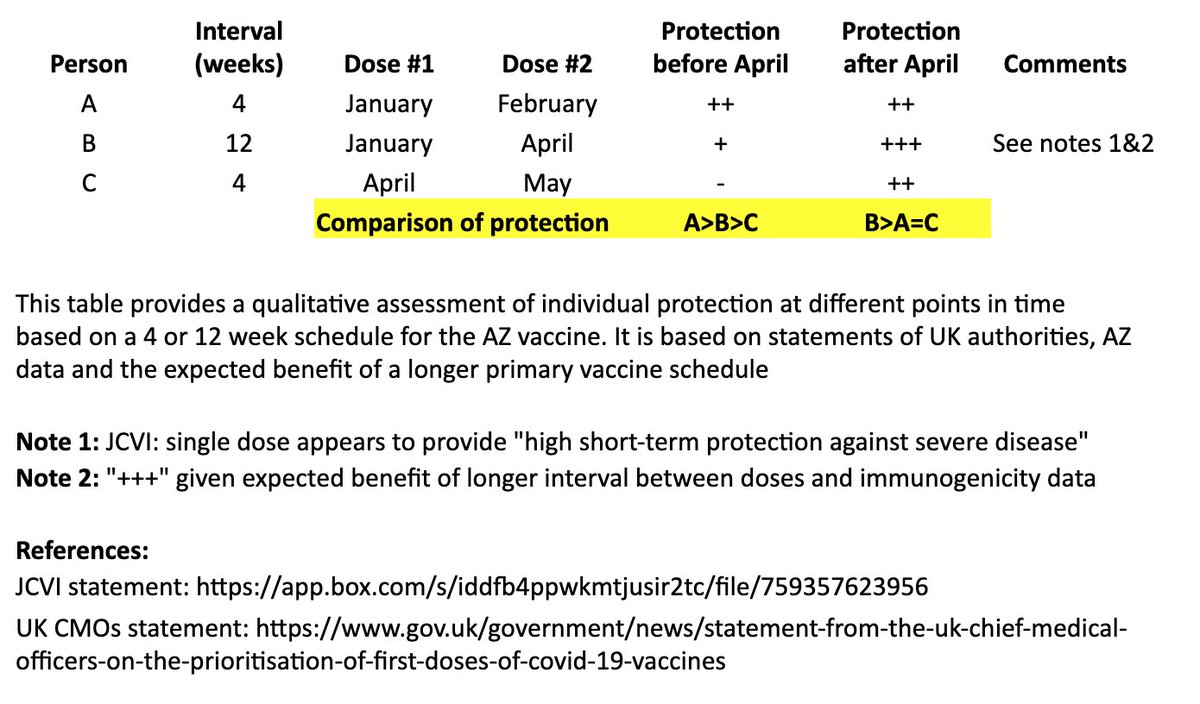
The bottom line is that “A” may see some protective benefit over “B” for two months (although “B” is expected to be protected against severe COVID during that time), but “B” is expected to have better long-term protection after they receive their second dose at 12 weeks. 9/ 
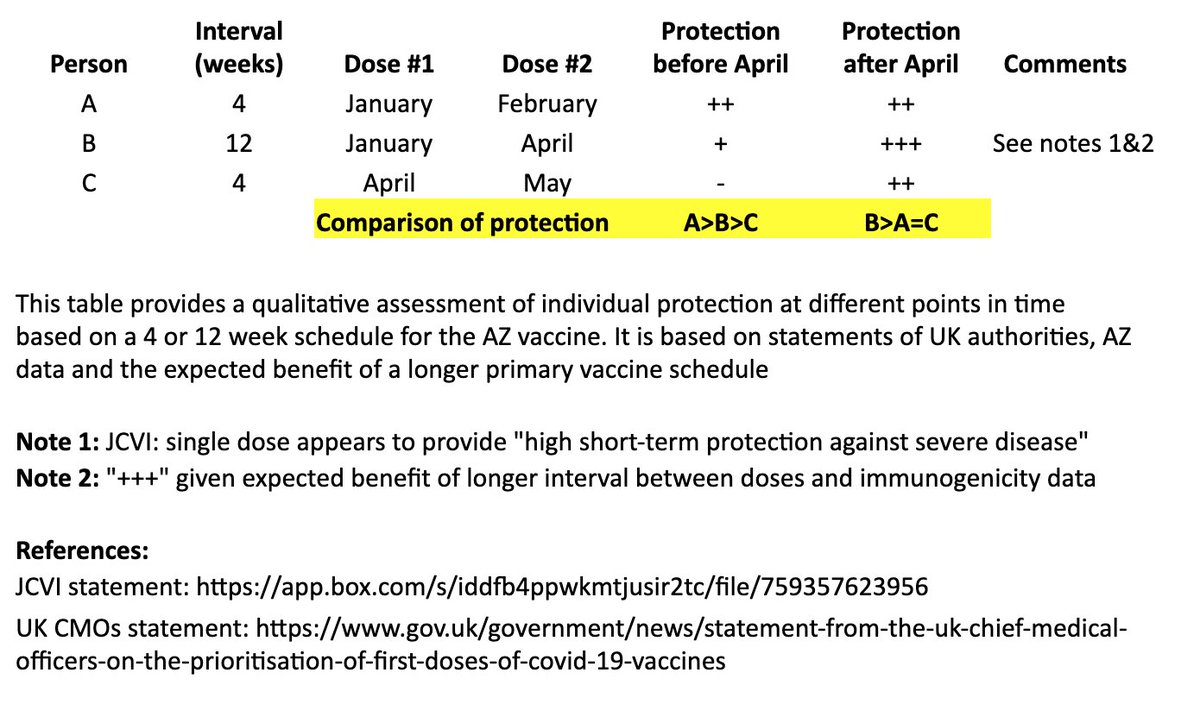
In other words, for the two months between “A” and “B’s” second dose: A>B>C
For the longer term, after “B” receives their second dose: B>A=C
10/
For the longer term, after “B” receives their second dose: B>A=C
10/
At a population level, this strategy could double the number of people immunized immediately while production ramps up. Many in category “C” could shift to “B.”
This assumes immunization programs are keeping up with the number of doses available. 11/
This assumes immunization programs are keeping up with the number of doses available. 11/
A few important caveats: (1) the assumption of sustained efficacy after the first dose is based on an exploratory analysis in the age 18-55 population. This needs to be confirmed in the older adult population. Exploratory analyses have limitations, esp if not pre-specified. 12/
(2) the assumption that a longer schedule (“B”) will result in a higher-quality, more durable immune response is based on immunogenicity data and experience with other vaccines. The efficacy impact will have to be confirmed. 13/
(3) we need to confirm that vaccine protection against the new strain is comparable after the first and second doses, given the affinity maturation expected with a second dose. 14/
Some virologists have raised the theoretical concern that a change in schedule could promote emergence of vaccine escape mutants. This is an important consideration that has implications for single-dose vaccine development and WHO’s minimum vaccine efficacy threshold of 50%. 15/
Again, this illustration is based on statements from UK authorities, AZ’s published data, and what is known from prior vaccine development. It is not a prediction of the future or what will happen to any individual, and is subject to updates as new data becomes available. 16/ 
I hope this helps people understand the practical ramifications of the UK recommendations and the pros/cons at individual and population levels. The urgency of the question is driven by the emergence of the new variant in the UK as well as the limited supply of vaccine. 17/
Thanks to our vaccine CMO, Dr. Gary Dubin for his input. More thoughts and links to others' views in the thread below.
I went through this exercise to clarify my own views and thought others might find it useful. Please take it in that spirit! 18/
I went through this exercise to clarify my own views and thought others might find it useful. Please take it in that spirit! 18/
https://twitter.com/rvenkayya/status/1345026156257550339?s=20
• • •
Missing some Tweet in this thread? You can try to
force a refresh



