Let me tell you an amazing story...
In the 1990s, a maverick breast surgeon at @TataMemorial (fresh from his return from the UK) stepped up to do research. Now, to understand the situation, you should go back 30 years, when research was not as big as it is now, and certainly not from surgeons.
Surgeons, and especially cancer surgeons, were renowned for their technical prowess, and their sheer bravado – "wherever the cancer, however advanced, I will take it out". So, our surgeon-researcher was ridiculed for even attempting clinical research
For a surgeon, he couldn’t have chosen a worse topic to research on: early detection; nothing to do with surgery, or even treatment. Remember, this was the 1990s. Cowboy surgery was celebrated, and research ridiculed
Being a breast surgeon, he was troubled with women consistently coming with advanced cancers, and he set out to see if he could work on picking them up at an earlier stage. But community-based early detection needs money, and he just didn’t have it.
He called up his friend who was a corporate leader with an Indian consumer company, and asked him to fund a pilot. Probably based more on friendship than his belief in the idea, the friend gave him a small grant
Our protagonist breast surgeon, accompanied by a couple of his co-workers go around the lanes of Parel (Mumbai) daily, examining women clinically to detect breast cancer early. They examine 4000 women, and find two cancers
By a strange twist of fate, the 1994 @uicc annual Congress was held in New Delhi by the @TataMemorial
Our maverick presented these results at the meeting. What followed subsequently would be unbelievable in today’s world
Our maverick presented these results at the meeting. What followed subsequently would be unbelievable in today’s world
A tall American gentleman walked up to our breast surgeon, congratulated him on the study and suggested he apply for a grant from the @NIH
At the time, our hero had no idea that the American gentleman was in charge of grant funding at the @NIH
At the time, our hero had no idea that the American gentleman was in charge of grant funding at the @NIH
To cut a long story short, the application forms arrived, the application was made (ambitiously, for breast and cervical cancer) , and our hero received an @NIH R01 grant! This is an extremely difficult grant to get...
Soon, he started a community-based cluster randomized trial evaluating the role of #VisualInspectionAceticAcid and #ClinicalBreastExamination for early detection of cervical and breast cancer respectively in 150,000 women between 35 and 64 years in the slums of Mumbai
The challenges were enormous: skeptical women (and skeptical families); the sheer logistics of screening 75000 women with #VIA and #CBE every 2 years for 8 years, and following up 150,000 women for 20 years; recording every cancer; recording every death
He had great support: his mentee (Dr Rajan Badwe, who went on to become the Director of the @TataMemorial), some young, idealistic preventive oncology physicians (Dr Gauravi Mishra & Dr S Shastri), over a 100 dedicated high school educated women as health workers
Relentlessly screening, recording, documenting, following up every one of these 150,000 women, over 20 years! And remember, these were Mumbai slums.. where migration was the rule rather than the exception
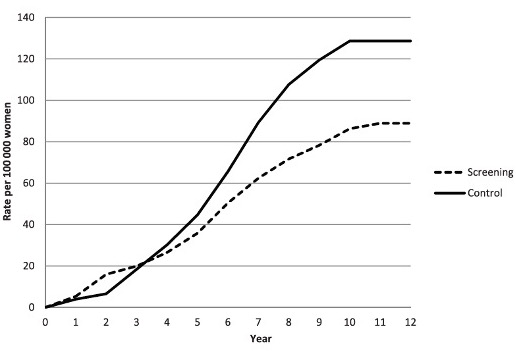
15 years later, the cervical cancer screening results came in – a 31% reduction in cervical cancer mortality, using a low-cost, low-tech method, which soon was adopted by several Indian states 
The cervical cancer mortality reduction was recognized and given the privilege of a plenary presentation at @ASCO ; to give context, 5 out of 35000+ abstracts are chosen for an @ASCO plenary talk 

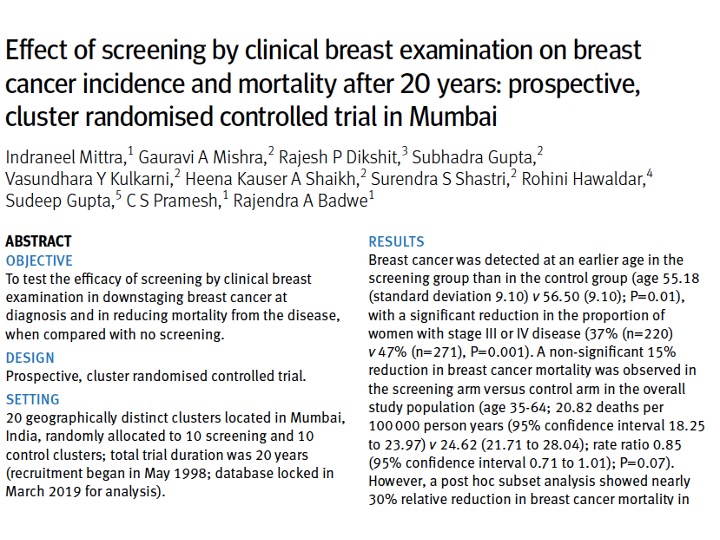
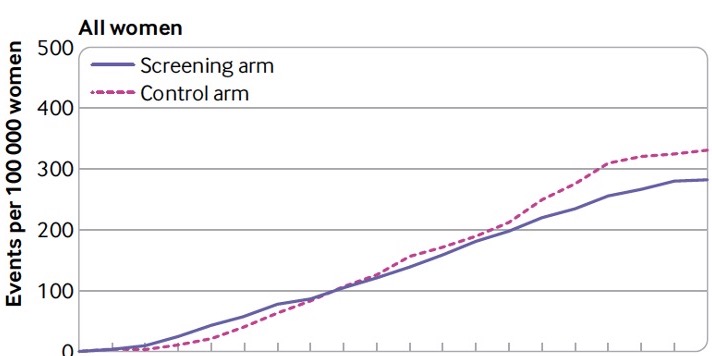
20 years later, the breast cancer results kicked in – a 15% (non-significant) reduction in breast cancer mortality in the study population, with a 30% breast cancer mortality reduction in women >50 years 



One man. With a vision. And the belief. Saving thousands of lives of women with breast and cervical cancer. Folks, salute the indomitable Prof Indraneel Mittra 

• • •
Missing some Tweet in this thread? You can try to
force a refresh