
🚨Interim results of @TogetherTrial of ivermectin and fluvoxamine for early treatment of #COVID_19 🚨
#ivermectin : no significant effect
#fluvoxamine: ↘️ risk of hospitalization by 31%
These important results deserve a🧵 1/n rethinkingclinicaltrials.org/news/august-6-…
#ivermectin : no significant effect
#fluvoxamine: ↘️ risk of hospitalization by 31%
These important results deserve a🧵 1/n rethinkingclinicaltrials.org/news/august-6-…
Patients with early #COVID_19 (e.g. <7days after onset of symptoms) and at least one risk factor were enrolled. 3/n 

#Ivermectin (0.4mg/kg for three days) was not found to have any effect.
Very high number of enrolled patients. N = 1,355 total. No statistically significant effect on risk of hospitalisation or mortality. 5/n
Very high number of enrolled patients. N = 1,355 total. No statistically significant effect on risk of hospitalisation or mortality. 5/n

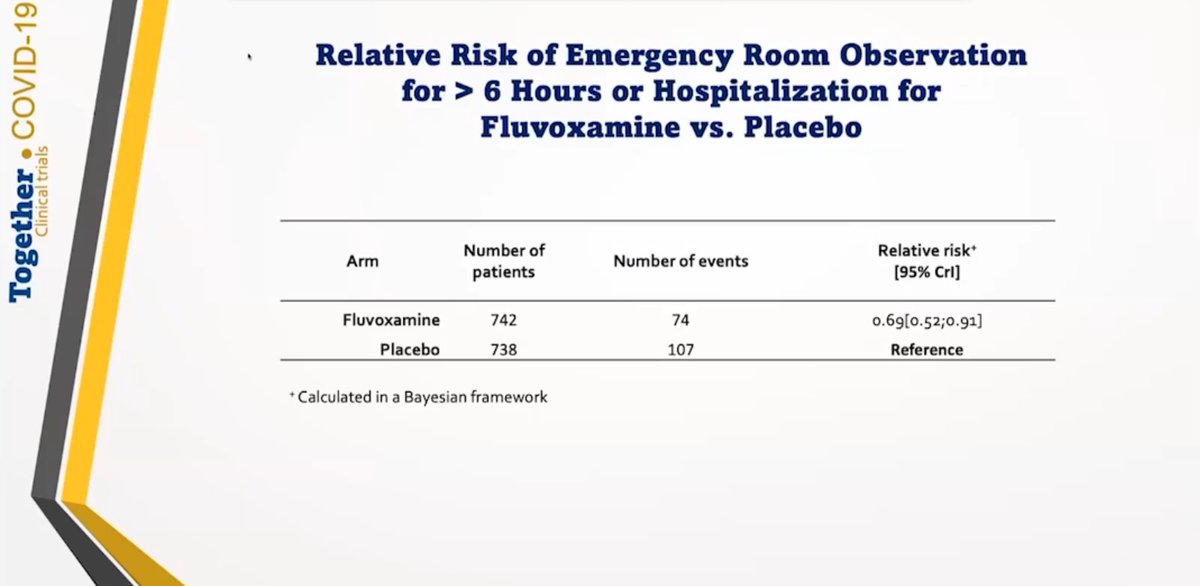
#Fluvoxamine (200mg/day for ten days) was found to reduce risk of hospitalisation by 31% (RR 0.69 [0.52;0.91].
Very high number of enrolled patients. N = 1,480 total. 6/n
Very high number of enrolled patients. N = 1,480 total. 6/n
Here are the results of fluvoxamine on mortality. Not statistically significant, but the trial was not powered to detect an effect on mortality. 7/n 

What next?
First, it is important to continue investigating why fluvoxamine would work... 8/n
First, it is important to continue investigating why fluvoxamine would work... 8/n
Fluvoxamine is a selective serotonin reuptake inhibitor. At least 3 putative MoAs:
▶️antiplatelet (depletes platelet serotonin)
▶️anti-inflammatory (sigma1 receptor agonist)
▶️antiviral (inhibits virus entry into cells through 'FIASMA' effect) 9/n pubmed.ncbi.nlm.nih.gov/33959018/
▶️antiplatelet (depletes platelet serotonin)
▶️anti-inflammatory (sigma1 receptor agonist)
▶️antiviral (inhibits virus entry into cells through 'FIASMA' effect) 9/n pubmed.ncbi.nlm.nih.gov/33959018/
Secondly, although fluvoxamine is a cheap generic drug, access in LMICs may be challenging (i.e. not on WHO Essential Medicines List). There is a need to evaluate more accessible drugs with similar characteristics.
#Fluoxetine is the best candidate. 10/n
#Fluoxetine is the best candidate. 10/n
https://twitter.com/julienpotet/status/1351814171315351554
Thirdly, now is the time for combinations. Investigators of the Together Trial recommend to assess effectiveness of fluvoxamine (or fluoxetine) + inhaled budesonide for early treatment of COVID-19. 11/n
Fourthly, and most importantly, decision-makers and regulators will need to decide if fluvoxamine should now receive emergency use approval for early treatment of COVID-19 in countries with low vaccination rates and raging epidemic waves. In which patients? 12/n
Finally, kudos👏👏👏 to all those who have relentlessly argued that #fluvoxamine was a promising candidate for #COVID_19. These include @AngelaReiersen, @EricLenze2, @boulware_dr, @HoertelN, @farid__jalali & the late @__ice9. 13/END
• • •
Missing some Tweet in this thread? You can try to
force a refresh









