Recently, we published the concluding part III of the #SPIRAL model, which we were working on since 2016.
Pathological T Helper Polarization Requires Pre-existing Cross-Reactive Memory T Cells
I'm going to explain the significance of this model.
preprints.org/manuscript/202…
Pathological T Helper Polarization Requires Pre-existing Cross-Reactive Memory T Cells
I'm going to explain the significance of this model.
preprints.org/manuscript/202…
In part I published in 2018, we introduced the SPIRAL model. It proposes that cross-reactivity is the basic unit that drives the evolution of adaptive immune system and it is thymus-derived regulatory T cells, Tregs, that rely on cross-reactive epitopes to control other T cells
We also proposed that thymus-derived #Foxp3 Tregs rely on #CrossReactive epitope derived from the friendly gut microbiota to survive and function in the periphery. So one of the main roles of gut microflora is to supply #Tregs with epitopes.
onlinelibrary.wiley.com/doi/10.1111/sj…
onlinelibrary.wiley.com/doi/10.1111/sj…
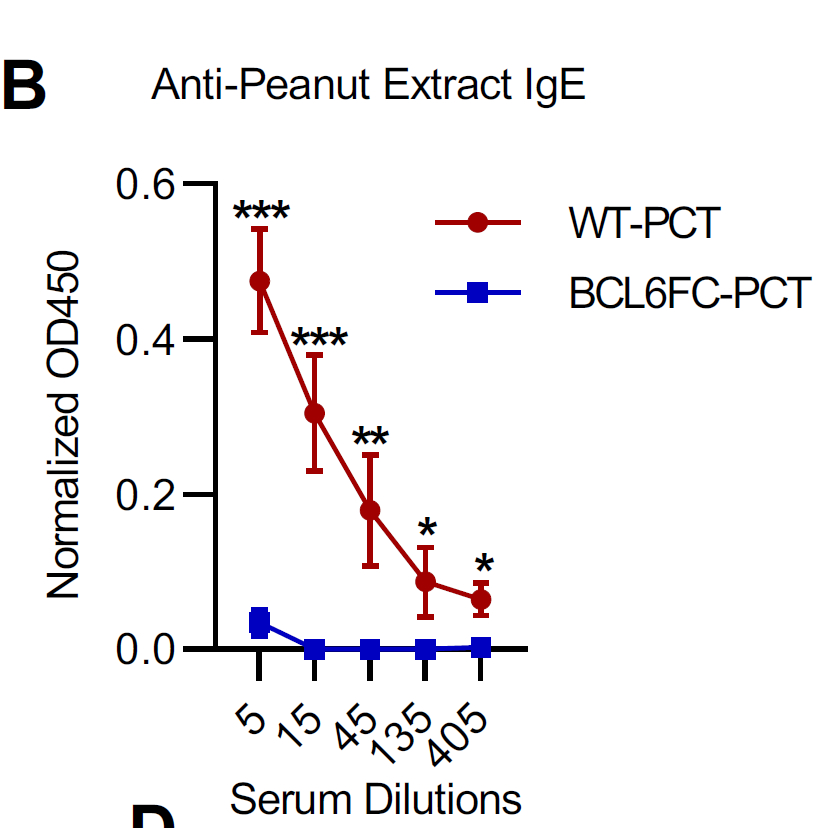
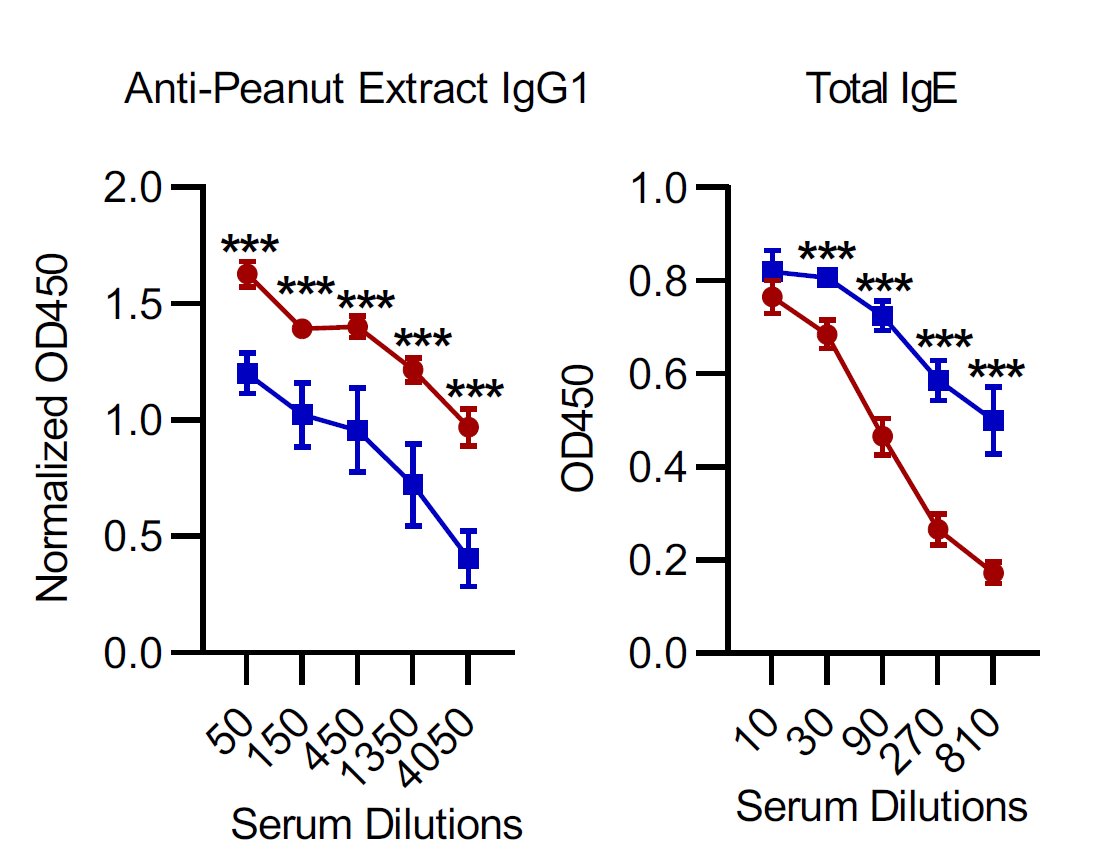
when we lose good microflora we lose the corresponding epitope-specific thymus-derived Tregs and that induces both allergies and autoimmunity but only if antigens that induce allergy or autoimmunity are cross-reactive.
in part II, we tackled the question of the origin of thymus-derived Tregs. They need epitopes from friendly microbiota, but Tregs need IL-2 too. Where is this IL-2 coming from and how? #CrossReactivity provided the answer here too.
We proposed that IL-2 is derived from T cells that express TCR that recognize similar epitopes as Tregs. In essence, each Treg clone has its own 'partner' IL-2-producing T cells that they exist in dyads, a functional unit, necessary for Treg survival.
Basically, Tregs need both microbiota-derived epitopes and IL-2 derived from T cells sharing TCR cross-reactivity. It is these IL-2-producing T cells that drive pathologies when we lose microbiota and lose Tregs.
in this, concluding part III of the SPIRAL model, we asked what is so special about these IL-2 producing T cells? We proposed that these IL-2 producing T cells that normally act as Treg 'partners' are in fact memory T cells and source of all pathological T helper polarizations.
In immunology, T helper differentiation and T helper polarization are used interchangeably. However, these are two different phenomena. T helper differentiation is a physiological process driven by the innate system from naive T cells while T helper polarization is a pathology &
requires the presence of #CrossReactive memory T cells. The innate signaling alone cannot induce T helper polarization. 

The polarized T helper response is a source of immune pathology such as allergies, ineffective immune response to pathogens. All these require #CrossReactivity and loss of Treg/microbiota axis.
why do we have allergies? there is nothing special about allergen except is it recognized by cross-reactive, pre-existing memory T cells and there is loss of 'partner' Treg/microbiota axis to keep them in check.
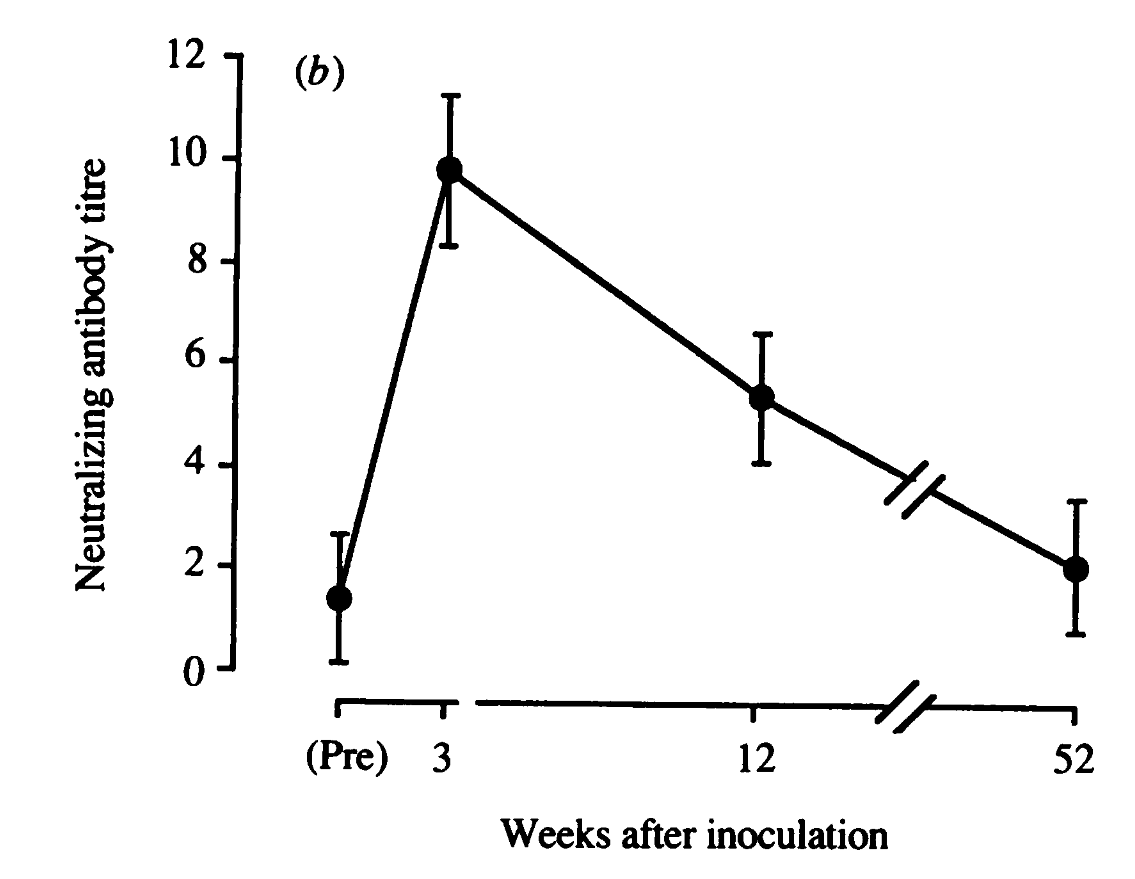
what is an ineffective immune response to pathogens? it is when a new pathogen re-activates pre-existing memory T cells via cross-reactivity and there is no 'partner' Treg/microbiota axis to stop it from polarization and inhibiting other naive T cells.
#OriginalAntigenicSin
#OriginalAntigenicSin
properly functioning adaptive immune system needs the full house of Tregs that relies on microbiota-derived epitopes and IL-2 produced by 'partner' memory T cells. It all depends on epitope #CrossReactivity. 

• • •
Missing some Tweet in this thread? You can try to
force a refresh