1/
Should we use #remdesivir (RDV) to treat high-risk outpatients with #SARSCoV2 #COVID19?
Here's #HowIReadThisPaper on the PINETREE trial by Gottleib et al in @NEJM
(Thread)
nejm.org/doi/full/10.10…
Should we use #remdesivir (RDV) to treat high-risk outpatients with #SARSCoV2 #COVID19?
Here's #HowIReadThisPaper on the PINETREE trial by Gottleib et al in @NEJM
(Thread)
nejm.org/doi/full/10.10…
2/
High-quality evidence suggests RDV shortens time to clinical improvement in hospitalized patients with #COVID19 and hypoxemia (ACTT-1).
Based on this, @NIH recommends RDV in hypoxemic inpatients:
covid19treatmentguidelines.nih.gov/management/cli…
However, its role in treating outpatients is unknown.
High-quality evidence suggests RDV shortens time to clinical improvement in hospitalized patients with #COVID19 and hypoxemia (ACTT-1).
Based on this, @NIH recommends RDV in hypoxemic inpatients:
covid19treatmentguidelines.nih.gov/management/cli…
However, its role in treating outpatients is unknown.
3/
What was studied?
Patients were randomized (double-blind) to receive IV RDV or placebo x 3 days
The primary outcome was the composite of COVID-related hospitalization or death from any cause by day 28.
What was studied?
Patients were randomized (double-blind) to receive IV RDV or placebo x 3 days
The primary outcome was the composite of COVID-related hospitalization or death from any cause by day 28.
4/
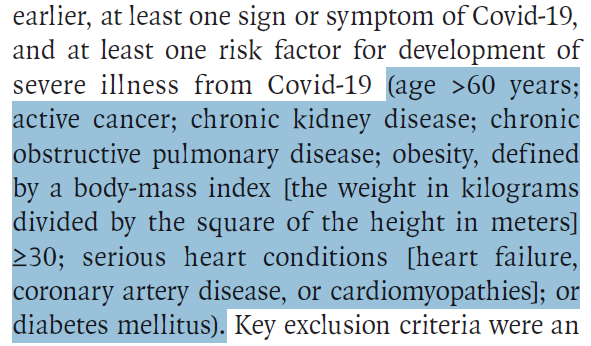
Who was studied?
562 non-hospitalized, unvaccinated patients
Age > 12 years
Sx onset < 7 days
>1 risk factor for severe disease: age >60, obesity, CAD, CVA, HTN, immunosuppression or cancer, CKD, DM2, CLD, COPD, sickle cell disease
Who was studied?
562 non-hospitalized, unvaccinated patients
Age > 12 years
Sx onset < 7 days
>1 risk factor for severe disease: age >60, obesity, CAD, CVA, HTN, immunosuppression or cancer, CKD, DM2, CLD, COPD, sickle cell disease
5/
~95% of patients were enrolled in the US.
Mean age: 50
Most common comorbidities: DM2, obesity, HTN
The study was done before the emergence of delta or omicron
~95% of patients were enrolled in the US.
Mean age: 50
Most common comorbidities: DM2, obesity, HTN
The study was done before the emergence of delta or omicron

6/
What were the main findings?
Primary outcome: HR = 0.13 (0.03 - 0.59)
Key secondary outcomes:
- Any medically attended visit by day 28: HR = 0.19 (0.07 - 0.56)
- No difference in viral load, symptoms, or safety signals
What were the main findings?
Primary outcome: HR = 0.13 (0.03 - 0.59)
Key secondary outcomes:
- Any medically attended visit by day 28: HR = 0.19 (0.07 - 0.56)
- No difference in viral load, symptoms, or safety signals

7/
This was a (very) positive study.
Should we worry that these results are too good to be true?
Let’s examine some sources of chance and bias to decide.
First, the primary outcome was changed - this can sometimes be a red flag.
Was this a problem here?
This was a (very) positive study.
Should we worry that these results are too good to be true?
Let’s examine some sources of chance and bias to decide.
First, the primary outcome was changed - this can sometimes be a red flag.
Was this a problem here?

8/
I don’t think so.
In response to comments from FDA, the investigators extended the duration of follow-up from 14 to 28 days.
All hospitalizations occurred before day 14, so this change was of no consequence.
Therefore, this is not a problem in my view.
I don’t think so.
In response to comments from FDA, the investigators extended the duration of follow-up from 14 to 28 days.
All hospitalizations occurred before day 14, so this change was of no consequence.
Therefore, this is not a problem in my view.
9/
Due to declining COVID19 infections, the trial was stopped before half the planned sample size was reached.
Usually, we worry about this leaving a study underpowered.
However, here we STILL see a large effect on hospitalizations.
This increases my confidence in the results.
Due to declining COVID19 infections, the trial was stopped before half the planned sample size was reached.
Usually, we worry about this leaving a study underpowered.
However, here we STILL see a large effect on hospitalizations.
This increases my confidence in the results.
10/
Further, a large drop in ANY medically attended visits for #COVID19 was shown.
This could have big implications for decongesting an already overburdened healthcare system.
Further, a large drop in ANY medically attended visits for #COVID19 was shown.
This could have big implications for decongesting an already overburdened healthcare system.

11/
Three notes of caution:
First, because no patients in either group died, we don’t know whether outpatient RDV has any effect on mortality.
For each of the three other NIH-recommended therapies for outpatients, fewer patients died on-treatment than in the control groups.
Three notes of caution:
First, because no patients in either group died, we don’t know whether outpatient RDV has any effect on mortality.
For each of the three other NIH-recommended therapies for outpatients, fewer patients died on-treatment than in the control groups.
12/
Second, there was no difference in nasopharyngeal viral load.
It would increase my confidence in the results to see changes in this secondary outcome that are mechanistically in-line with how we expect an antiviral to work.
The absence of a difference here is unexplained.
Second, there was no difference in nasopharyngeal viral load.
It would increase my confidence in the results to see changes in this secondary outcome that are mechanistically in-line with how we expect an antiviral to work.
The absence of a difference here is unexplained.
13/
Finally, the % of patients with positive SARS-CoV2 antibodies (indicating prior infection) was not reported.
Subgroup analysis by prior infection would help us judge whether these results are likely to generalize to people with prior infection, or who have been vaccinated.
Finally, the % of patients with positive SARS-CoV2 antibodies (indicating prior infection) was not reported.
Subgroup analysis by prior infection would help us judge whether these results are likely to generalize to people with prior infection, or who have been vaccinated.
14/
Despite these concerns, RDV has few risks.
It is also the most widely available of any of the four NIH-recommended treatments for high-risk outpatients.
Given these results, I believe strategies to overcome barriers to 3-day outpatient IV administration are warranted.
Despite these concerns, RDV has few risks.
It is also the most widely available of any of the four NIH-recommended treatments for high-risk outpatients.
Given these results, I believe strategies to overcome barriers to 3-day outpatient IV administration are warranted.
15/
Bottom line:
RDV led to a large reduction in hospitalizations and medically-attended visits overall for #COVID19 by day 28 in high-risk outpatients; however, we don’t know if it has any effect on mortality.
(End)
Bottom line:
RDV led to a large reduction in hospitalizations and medically-attended visits overall for #COVID19 by day 28 in high-risk outpatients; however, we don’t know if it has any effect on mortality.
(End)
• • •
Missing some Tweet in this thread? You can try to
force a refresh