1/As promised & after reading $NTLA latest Q1 2022 financial report here is my analysis regarding @intelliatx latest corporate status. I have focused only on the main issues that I found to be the most interesting & relevant. #CRISPR #GeneEditing #BioTech #FinTwit #Genomics👇 

2/NTLA-2001 is the most advanced In-Vivo #CRISPR/#Cas9 #GeneEditing program in $NTLA portfolio & is aimed to treat Transthyretin Amyloidosis - (ATTR). NTLA-2001 could potentially halt & reverse the disease in a “one time” treatment by knocking out the TTR gene with a single dose 

3/In February $NTLA has presented an updated clinical data from its Phase 1 study of NTLA-2001 which included 15 patients with ATTR amyloidosis. IMO NTLA-2001 readout looks promising & here is a summary that I wrote shortly after @intelliatx’s presentation of NTLA-2001👇
https://twitter.com/yaireinhorn/status/1498709922984472581
4/NTLA-2002 is aimed to knock out the KLKB1 gene in the #liver thus reducing total plasma kallikrein protein & activity, a key mediator of #HAE - hereditary #angioedema. In 12/1/21 $NTLA has dosed the 1ST patient & will publish interim data from the Phase 1/2 study in 2H of 2022. 

5/Two more In-Vivo programs are $NTLA 3001 & 2003: NTLA-3001 is aimed for AATD-associated #Lung disease & IND will be filled during 2023. NTLA-2003 is aimed for AATD-associated #Liver disease & @intelliatx is advancing towards IND-enabling activities for this program as well. 

6/NTLA-5001 is an autologous T-cell therapy aimed for the treatment of all genetic subtypes of #AML. In March @intelliatx announced that the first patient was dosed in its Phase 1/2a study & that @US_FDA has granted orphan drug designation to NTLA-5001 for the treatment of #AML👇 

7/IMO the most important development was the designation of NTLA-6001 - an allogeneic CAR-T program for treating hematologic #cancer including Hodgkin #lymphoma (cHL). NTLA-6001 is the first program that was developed using @intelliatx’s proprietary cell engineering platform👇
https://twitter.com/yaireinhorn/status/1525511525846458368
8/One of @intelliatx’s major strengths is its multiple collaborations: 1)acquisition of Rewrite 2)@OnkTherapeutics - the co - development of #CRISPR-edited NK #CellTherapy for #Cancer. 3)@KyvernaT - the development of KYV-201 - a CD19 CAR-T cell for an #autoimmune diseases.👇 

9/As of 3/31/22 $NTLA had capital resources of $994.7M & R&D expenses of $133.1M. IMO although the cash burn has increased by $94M, @intelliatx cash position of $994M will enable it to both continue to develop its pipeline & to initiate additional acquisitions & collaborations. 

10/Upcoming Milestones:
1.NTLA-2001 - ATTR:
ATTRv-PN arm - Phase 1 data in June 2022
ATTR-CM arm - Phase 1 data in 2H 2022
2.NTLA-2002 - HAE:
Phase 1/2 data in 2H 2022 3.NTLA-3001 - AATD:
File an IND in 2023
4.NTLA-5001 - AML:
Enrolment of patients in Phase 1/2a during 2022
1.NTLA-2001 - ATTR:
ATTRv-PN arm - Phase 1 data in June 2022
ATTR-CM arm - Phase 1 data in 2H 2022
2.NTLA-2002 - HAE:
Phase 1/2 data in 2H 2022 3.NTLA-3001 - AATD:
File an IND in 2023
4.NTLA-5001 - AML:
Enrolment of patients in Phase 1/2a during 2022

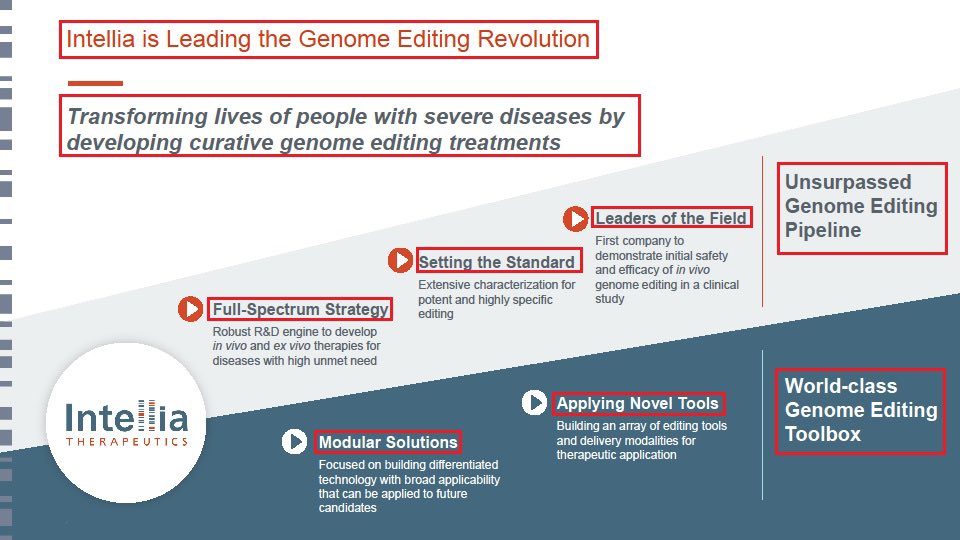
11/@intelliatx is definitely an impressive & promising #GeneEditing company. Both $NTLA proprietary pipeline & its systematic strategy of collaborations & acquisitions are the key factors which defines $NTLA as a leading #CRISPR and #GeneEditing company. 
on a personal note: one of my main interests in $NTLA was its collaboration with @SparingVision - aimed to develop #Ocular #GeneEditing platforms for the treatment of #IRDs. As Of Q1 22 no progress was published yet & I eagerly await to see what progress this agreement will bring
https://twitter.com/yaireinhorn/status/1448323985771483136
And as always please feel free to share and retweet so that those on @Twitter or #FinTwit who are interested in #GeneEditing, #CRISPR, #BioTech & #Genomics will get this relevant data. Enjoy the rest of your #Weekend!
• • •
Missing some Tweet in this thread? You can try to
force a refresh