1) Welcome to a new #accredited #tweetorial, "Utilizing Immunohistochemistry Testing, Biomarkers, and Targeted Therapeutics to Optimize Outcomes in Patients with NSCLC," featuring the highlights of a symposium presented at the #ESMOIO22 congress.
@myESMO #LCSM #FOAMed
@myESMO #LCSM #FOAMed

2) The faculty for this outstanding program were @peters_solange (Chair) 🇨🇭, @HosseinBorghaei 🇺🇸, Natasha Leighl MD 🇨🇦, and @dplanchard 🇫🇷. A truly international roster of experts in #oncology!
Don't miss prior accredited courses in this space at oncologytweetorials-ce.com/category/lung-….
Don't miss prior accredited courses in this space at oncologytweetorials-ce.com/category/lung-….
3) This program is supported by an educational grant from Sanofi. Statement of accreditation and author disclosures are at oncologytweetorials-ce.com/disclosures/.
4) Our understanding and characterization of various subtypes of #LCSM adenocarcinoma continues to expand! This potentially allows for more targeted and safer therapy. Newer markers such as #CEACAM5 are emerging, expanding treatment options ! 



5) Recent data on #CEACAM5-Targeted Therapeutics were discussed by @NarjustFlorezMD and @ShrutiPatelMD in a prior #tweetorial, still available for 🆓credit, at oncologytweetorials-ce.com/lungcancer_esm….
6) #Platinum-based chemotherapy has historically been standard #1L for #mNSCLC,
BUT
➡️ Responses only in 15–30%
➡️ Relatively short interval until progression.
More recently, #immunotherapy has emerged as a promising alternative ✔️
BUT
➡️ Responses only in 15–30%
➡️ Relatively short interval until progression.
More recently, #immunotherapy has emerged as a promising alternative ✔️

7) With #immunotherapy, 5y survival advanced NSCLC increased from 4.7 to 16% in 🇺🇸 in 2018 (🔓 pubmed.ncbi.nlm.nih.gov/29570421/). An #RCT ➡️ #nivolumab tx improved long-term OS rate & achieved durable responses in a proportion of patients with pretreated advanced #NSCLC. 

8) Immune therapies exploit the host immune system to monitor & destroy cancer cells via the upregulation of key immune checkpoints; at present, #NSCLC immunotherapy mainly refers to immune checkpoint inhibitors (#ICIs). @NCI 
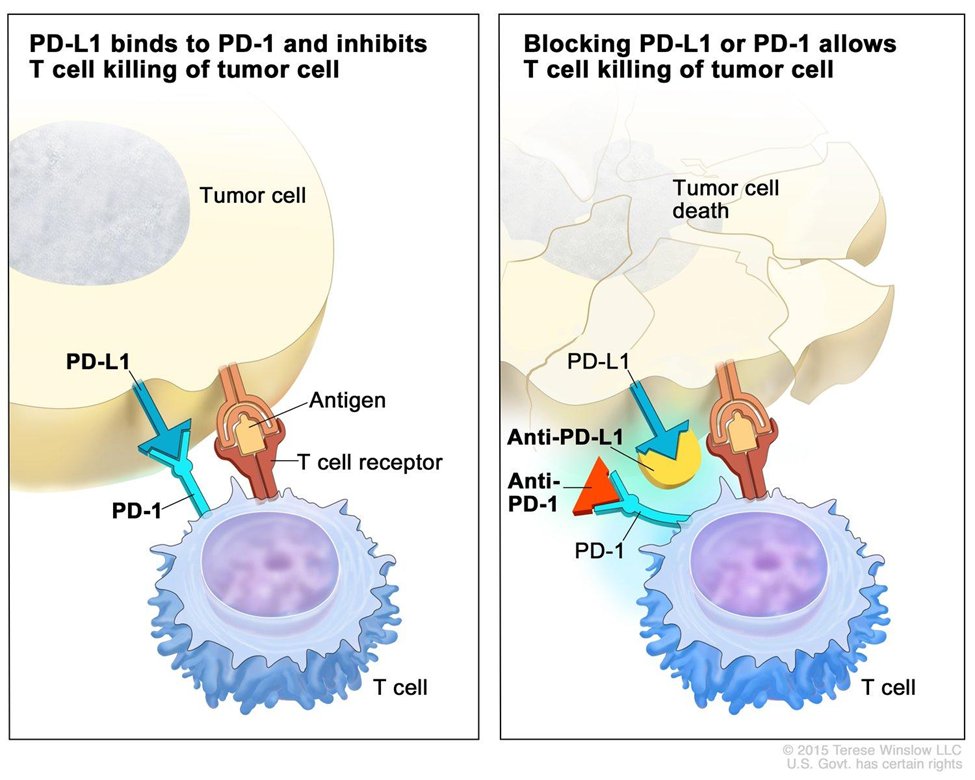
9) What biomarker is currently used in treatment decision making for 1L #mNSCLC without oncogenic drivers?
10) Currently, programmed death-ligand 1 (#PDL1) expression is the only clinically validated biomarker for selecting patients for tx with an #ICI. 
11) #PDL1 expression is best measured by immunohistochemistry (#IHC), which requires a surgical biopsy. Despite invasiveness, this is preferred to #liquid_biopsy. 





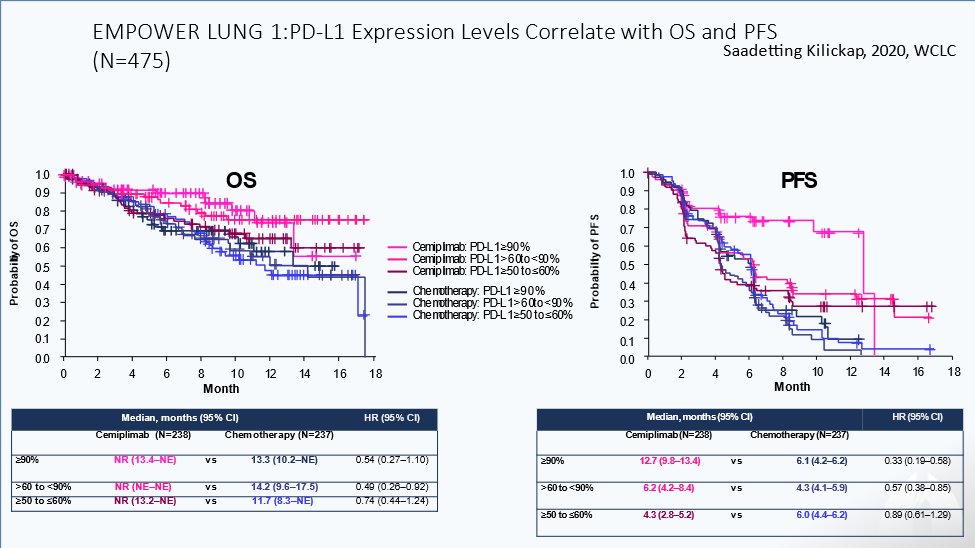
12) Let’s discuss treatment #selection! What level of PD-L1 expression is currently preferred for use of single agent immunotherapy?
13) It’s > 50%! PD-L1 testing 🔭 subgroup of pts who can benefit from #ICIs alone or in combo with #chemotherapy
👉The higher the level of PD-L1 expression, the better the chance for clinical efficacy
👉Tumor Mutation Burden #TMB is an effective marker for following tx success

👉The higher the level of PD-L1 expression, the better the chance for clinical efficacy
👉Tumor Mutation Burden #TMB is an effective marker for following tx success


14) Recently, we have seen major progress from targeting #NSCLC beyond chemotx (& PD-L1) w/signal transduction in oncogene addicted NSCLC:
👉Small molecule inhibitors, antibodies, ADCs
👉EGFR (inc exon20), BRAF, MET, KRAS, ERBB2
👉ALK, ROS1, NTRK, RET, (NRG1…)
👉Small molecule inhibitors, antibodies, ADCs
👉EGFR (inc exon20), BRAF, MET, KRAS, ERBB2
👉ALK, ROS1, NTRK, RET, (NRG1…)
15a) It's not just alphabet soup 🔤! Let's look at recent progress in data for #mNSCLC with targeting EFGR variants: @EGFRResisters 



15b) Epidermal growth factor receptor-targeting tyrosine kinase inhibitors (#EGFR #TKIs) are the standard of care for patients with EGFR-mutated #LCSM. While EGFR TKIs have initially high response rates, resistance constitute a major challenge to longitudinal tx. 

16) What about targeting #ALK fusions? The ALK locus is prone to translocation, & detection of ALK rearrangements is widely recognized in #LCSM. Different methods for detection include #IHC, fluorescence in situ hybridization #FISH, and next-generation sequencing #NGS. 



17) Smaller proportions of patients are impacted #mNSCLC driven by #ROS1 fusions, #BRAF mutations, #NTRK fusions, and #RET fusions, where we have witnessed good progress in treatments: 







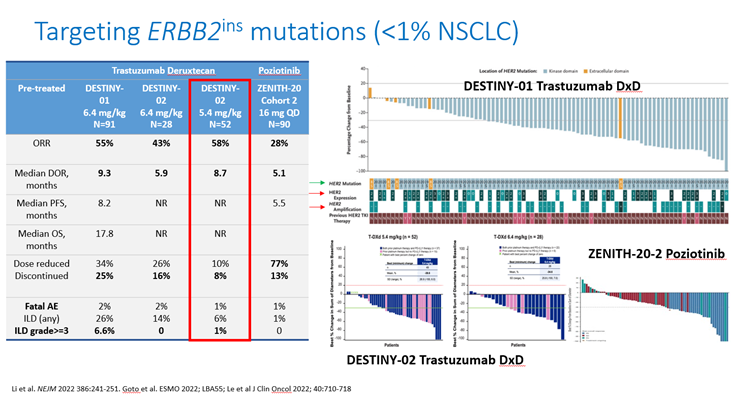
18) Similarly #MET skipping mutations, #KRAS skipping mutations (@KRASKickers!), and #ERBB2 mutations are now targetable for #mNSCLC #LCSM: 






19a) We mentioned Anti-#CEACAM5 therapy above . . . CEACAM5 is a #glycoprotein implicated in a variety of oncogenic activities, and was first recognized as a marker for #coloncancer. 



19b) Findings support #CEACAM5 as a potential therapeutic target and clinical development of #ADCs
🫁 CEACAM5 is preferentially expressed in #NSQ #NSCLC rather than in SQ NSCLC
🫁 #Tusamitamab_ravtansine is the first CEACAM5 emaytansinoid ADC to be evaluated in human subjects

🫁 CEACAM5 is preferentially expressed in #NSQ #NSCLC rather than in SQ NSCLC
🫁 #Tusamitamab_ravtansine is the first CEACAM5 emaytansinoid ADC to be evaluated in human subjects


19c)
🫁 Most frequent limiting dose toxicity was #keratopathy
Ongoing trials include Ph 2 studies with tusamitamab ravtansine in combination w/ #pembrolizumab (NCT04524689), or #ramucirumab (NCT04394624), and a phase III trial that compares tusamitamab ravtansine w/ #docetaxel
🫁 Most frequent limiting dose toxicity was #keratopathy
Ongoing trials include Ph 2 studies with tusamitamab ravtansine in combination w/ #pembrolizumab (NCT04524689), or #ramucirumab (NCT04394624), and a phase III trial that compares tusamitamab ravtansine w/ #docetaxel
20) It is an exciting time of rapidly expanding options for treatment of #mNSCLC. Which markers will have SUCCESS in improving outcomes for patients as we move forward? 

21) Thank you for following this 🧵! You can now claim 0.5hr 🆓CE/#CME by pointing your 🖱️ to oncologytweetorials-ce.com/lungcancer_esm…. And please follow us here at @onc_ce for more expert education in current topics in #oncology, delivered wholly on Twitter!
🙏@ADesaiMD for editorial support
🙏@ADesaiMD for editorial support
• • •
Missing some Tweet in this thread? You can try to
force a refresh




