#Paxlovid effect on reducing #COVID19 hospitalizations in vaccinated population finally revealed to the public by FDA. #IDTwitter
0.9% (3/317) paxlovid vs. 2.2% (7/314) placebo
fda.gov/media/166197/d…
0.9% (3/317) paxlovid vs. 2.2% (7/314) placebo
fda.gov/media/166197/d…

The relative risk reduction (RRR) of #covid19-related hospitalization was 57.5% (95%CI, -63% to 89%) with #Paxlovid in vaccinated high risk population.
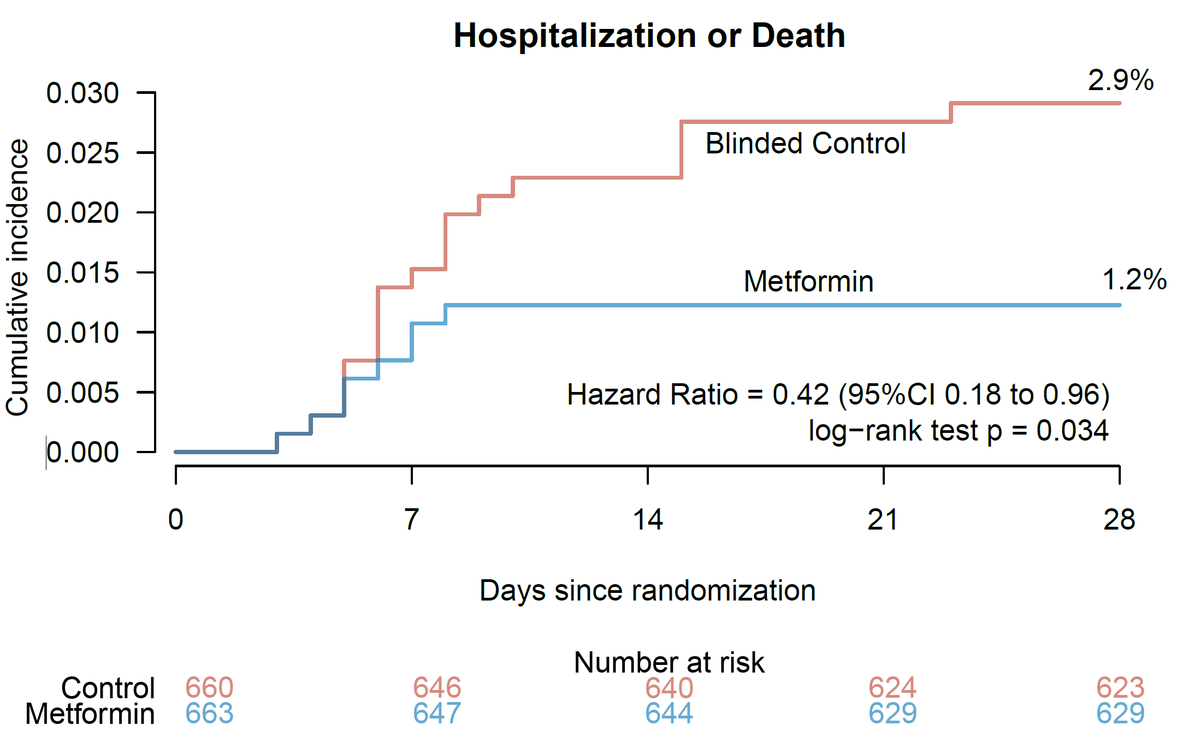
Will point out that the the Hazard Ratio is also 58% for $1 of #metformin.
Will point out that the the Hazard Ratio is also 58% for $1 of #metformin.
If one combines the EPIC-SR vaccinated + EPIC-HR seropositive groups together, then risk of #covid19 hospitalization was:
▪️ 0.5% (4/807) with #paxlovid
▪️ 1.9% (15/791) with placebo
Relative RIsk Reduction = 74% (95%CI, 22%-91%)
Number Needed to Treat = 71 (95%CI, 40-300)
▪️ 0.5% (4/807) with #paxlovid
▪️ 1.9% (15/791) with placebo
Relative RIsk Reduction = 74% (95%CI, 22%-91%)
Number Needed to Treat = 71 (95%CI, 40-300)
This last combined group is effectively the current population of seropositive persons with vaccination or prior infection.
At a NNT of 71, this equates to a cost to prevent 1 hospitalization of: $38k (95%CI, 21.5k to $159k).
In 2020, hospitalization without ICU cost $26,952.
At a NNT of 71, this equates to a cost to prevent 1 hospitalization of: $38k (95%CI, 21.5k to $159k).
In 2020, hospitalization without ICU cost $26,952.
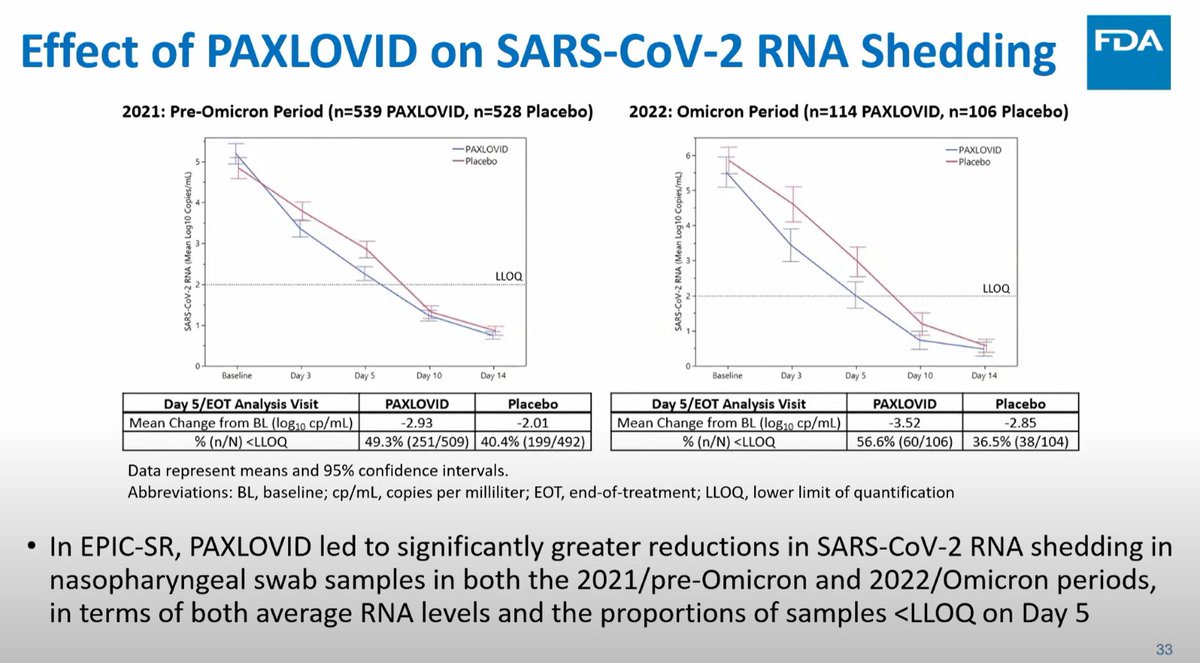
There was a viral effect in the EPIC-SR trial in vaccinated high risk persons, with an 0.84 log10 copies/mL better reduction with #paxlovid than placebo.
In seropositive EPIC-HR, paxlovid was 0.47 log10 copies/mL better than placebo.
In seropositive EPIC-HR, paxlovid was 0.47 log10 copies/mL better than placebo.

This was the day 5 anti-viral effect.
By Day 10, there was minimal difference in viral loads with paxlovid over placebo.
(In contrast metformin continues to have an antiviral effect at day 10... just saying).
By Day 10, there was minimal difference in viral loads with paxlovid over placebo.
(In contrast metformin continues to have an antiviral effect at day 10... just saying).

As very well reported, #paxlovid was associated with higher levels of viral rebound (meaning undetectable at day 5 and detectable virus at day 10 or 14).
Viral rebound occurred in EPIC-SR during the Omicron era (but not during the delta era).
Viral rebound occurred in EPIC-SR during the Omicron era (but not during the delta era).
https://twitter.com/PaulSaxMD/status/1636025926101405698
Detectable virus was statistically less common in EPIC-HR trial with #paxlovid vs placebo. The effect was present but, I woudl say relatively modest.
Abs Risk Reduction over placebo
Day5 = 3.7% (95%CI, -0.8% to 8.2%)
Day10 = 7.3% (95%CI, 3.2% to 11.3%)
Abs Risk Reduction over placebo
Day5 = 3.7% (95%CI, -0.8% to 8.2%)
Day10 = 7.3% (95%CI, 3.2% to 11.3%)

Effect of #Metformin on #SARSCoV2 viral load
Abs Risk Reduction over placebo
Day5 = 4.7% (95%CI, -2.0% to 11.3%)
Day10 = 8.3% (95%CI, 2.9% to 13.7%)
Thus $1 has larger viral effect for %undetectable at Day10 than $530 of paxlovid.
Abs Risk Reduction over placebo
Day5 = 4.7% (95%CI, -2.0% to 11.3%)
Day10 = 8.3% (95%CI, 2.9% to 13.7%)
Thus $1 has larger viral effect for %undetectable at Day10 than $530 of paxlovid.

Not present are the %undetectable over time in EPIC-SR standard risk group.
Also risk of hospitalization in the unvaccinated, standard risk group was:
▪️ 0.9% (2/223) paxlovid
▪️ 1.4% (3/214) placebo
Also risk of hospitalization in the unvaccinated, standard risk group was:
▪️ 0.9% (2/223) paxlovid
▪️ 1.4% (3/214) placebo
No difference in allievating symptoms faster in standard risk group.
No difference existed in ongoing symptoms at Day 28 (i.e. possible #longcovid) 72.8% paxlovid vs. 73.7% placebo in EPIC-SR trial.
But these EPIC trials did not follow participants beyond 28 days.
No difference existed in ongoing symptoms at Day 28 (i.e. possible #longcovid) 72.8% paxlovid vs. 73.7% placebo in EPIC-SR trial.
But these EPIC trials did not follow participants beyond 28 days.

EPIC-PEP Trial on post-exposure prophylaxis:
Among those wth neg #SARSCOV2 PCR result at baseline, event rates were:
▪️ 2.6% (22/844) in the PAXLOVID 5-day arm
▪️ 2.4% (20/830)in the PAXLOVID 10-day arm
▪️ 3.9% (33/840) with placebo
And appears ~8.1% (222/2736) PCR+ at baseline.
Among those wth neg #SARSCOV2 PCR result at baseline, event rates were:
▪️ 2.6% (22/844) in the PAXLOVID 5-day arm
▪️ 2.4% (20/830)in the PAXLOVID 10-day arm
▪️ 3.9% (33/840) with placebo
And appears ~8.1% (222/2736) PCR+ at baseline.
Safety.
Overall rates of hospitalization less different with #Paxlovid.
Serious AEs - likely hospitalizations:
EPIC-SR:
▪️8 (1.5%) pax (n=3 non-covid) vs
▪️11 (2.1%) placebo (n=1 non-covid).
EPIC-PEP:
⬆️SAES (hospitalizations) occurred in 5d prophylaxis (0.3%) vs placebo (0.2%)
Overall rates of hospitalization less different with #Paxlovid.
Serious AEs - likely hospitalizations:
EPIC-SR:
▪️8 (1.5%) pax (n=3 non-covid) vs
▪️11 (2.1%) placebo (n=1 non-covid).
EPIC-PEP:
⬆️SAES (hospitalizations) occurred in 5d prophylaxis (0.3%) vs placebo (0.2%)

What were the SAEs in the EPIC-SR trial are not stated by the FDA (i.e. hidden from the public). Presumably these are non-covid hospitalizations which occured through Day 34.
EPIC-SR:
All-cause hospitalization ARR = 0.6% at NNT = 166 at cost of $88k to prevent 1 hospitalization
EPIC-SR:
All-cause hospitalization ARR = 0.6% at NNT = 166 at cost of $88k to prevent 1 hospitalization
This is based on the difference between SAEs listed in Table 12 & Table 4 #COVID19 hospitalizations or all-cause deaths.
EPIC-SR Table 4 do not include the 287 enrolled in 2022. Hospitalization more like:
▪️ 1.17% (8/675) #paxlovid
▪️1.64% (11/661) placebo
ARR=0.5%, NNT=215
EPIC-SR Table 4 do not include the 287 enrolled in 2022. Hospitalization more like:
▪️ 1.17% (8/675) #paxlovid
▪️1.64% (11/661) placebo
ARR=0.5%, NNT=215

That @US_FDA deliberately excludes the n=287 enrolled in EPIC-SR trial during Omicron seems purposefully deceptive.
The 287 are not counted towards denominator of hospitalization (not tough to add) nor safety data presented.
Were excess SAEs occuring with paxlovid in 2022?
The 287 are not counted towards denominator of hospitalization (not tough to add) nor safety data presented.
Were excess SAEs occuring with paxlovid in 2022?
If one counts all SAEs in EPIC-SR (assuming these to be all-cause hospitalizations or life threatening AEs) and including the n=287 enrolled in 2022, then the: relative risk reduction is ~ 28% (95%CI, -77% to 71%) for all-cause hospitalization with #paxlovid. #COVID19 #IDTwitter
So what's my overall take away?
#Paxlovid is useful for high risk elderly or immunocompromised.
Actual absolute high risk, not the CDC basket of higher relative risk.
If age <60yo and with a normal immune system, likely near zero benefit if vaccinated/survived prior infection.
#Paxlovid is useful for high risk elderly or immunocompromised.
Actual absolute high risk, not the CDC basket of higher relative risk.
If age <60yo and with a normal immune system, likely near zero benefit if vaccinated/survived prior infection.
Pfizer's ADMAC slide on for COVID-related hospitalization or death.
This says "all cause" hospitalization but that does not match the SAE count in EPIC-SR (n=8 and n=11). Curious as to what were the SAEs?
This says "all cause" hospitalization but that does not match the SAE count in EPIC-SR (n=8 and n=11). Curious as to what were the SAEs?

SAEs pooled together quite cleverly for EPIC-HR and EPIC-SR. A good way to hide the EPIC-SR SAE data. 

Pooled SAE data from EPIC-HR and EPIC-SR. Somehow three of the SAEs in this list with paxlovid were deemed not COVID-related. 

Which group is #paxlovid most beneficial? Very clear. This is a medicine which was highly benefical in 2021. 

EPIC-HR paxlovid effect on hopsitalization/death in Obese (BMI>=30). Note the effect in the seropositive. 

#Paxlovid Effect in Seropositive for reducing #COVID19 hospitalization/death in EPIC-HR "high risk" trial. 

• • •
Missing some Tweet in this thread? You can try to
force a refresh