Our latest study to understand the dynamic regulation of #interferon (#IFN) and #SARSCoV2 infection.
🧵summarizing what we found and what needs to be done to understand the dynamic interplay of infection and #IFN regulation. @iScience_CP #COVID19
cell.com/iscience/fullt…
🧵summarizing what we found and what needs to be done to understand the dynamic interplay of infection and #IFN regulation. @iScience_CP #COVID19
cell.com/iscience/fullt…
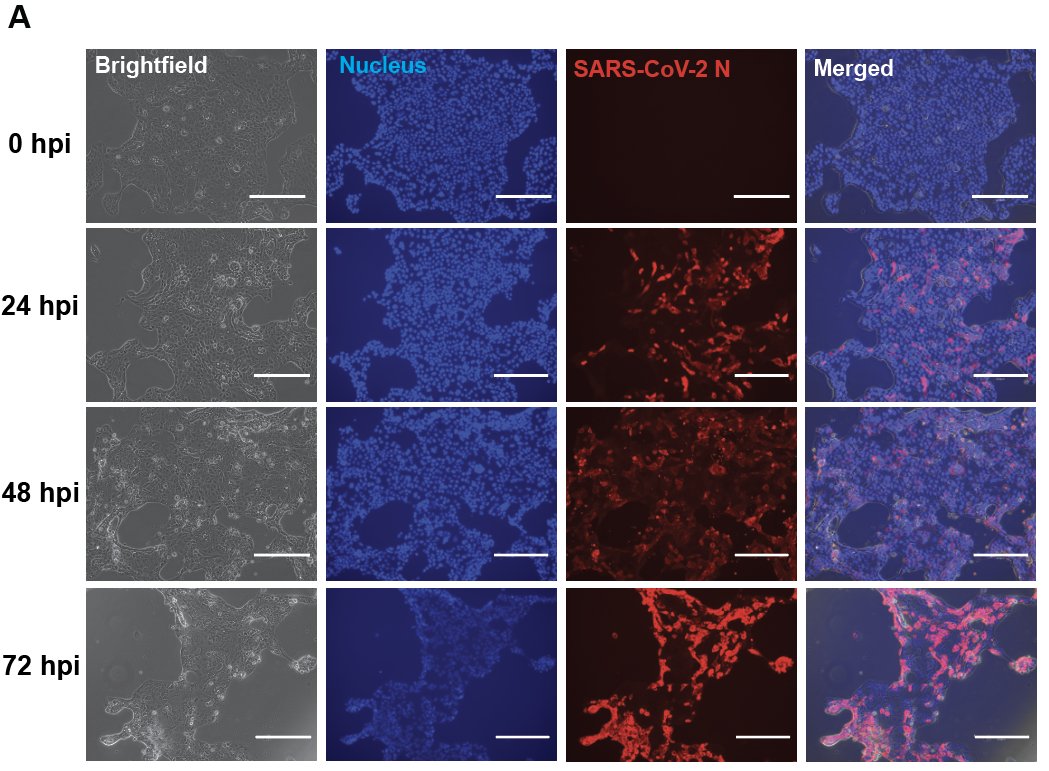
We infected human airway cells (Calu3) with #SARSCoV2, and performed a time-series #RNAseq analysis to discover global transcriptional responses. We saw expression of #IFN (beta and lambda) and IFN stimulated genes in these cells. 

#SARSCoV2 infection has an additive effect on the amount of #IFNbeta expression and downstream signaling induced by a synthetic stimulator of these responses (polyI:C). 
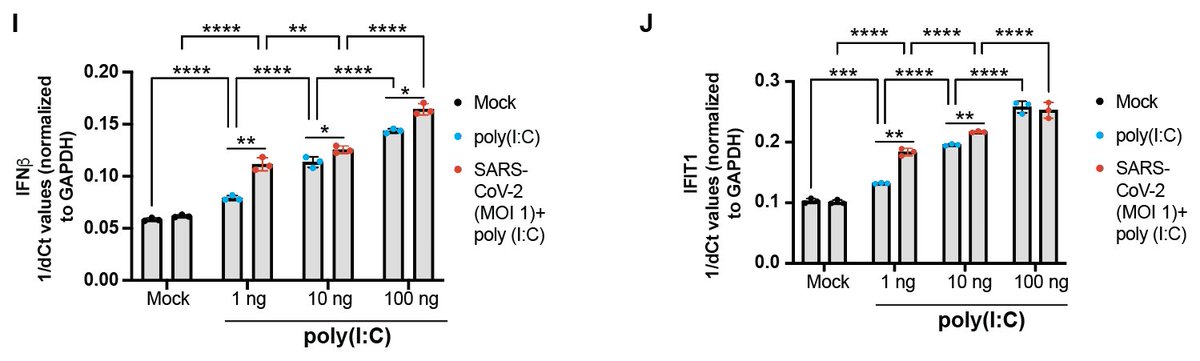
We also found that #SARSCoV2 infection (multiplicity of infection 1) has an additive effect on the amount of downstream #IFN signaling (i.e. ISG production) induced by recombinant type I IFNs. 

Next, we looked at the cytokine profiles of healthy individuals vs. patients with moderate or severe #COVID19. 
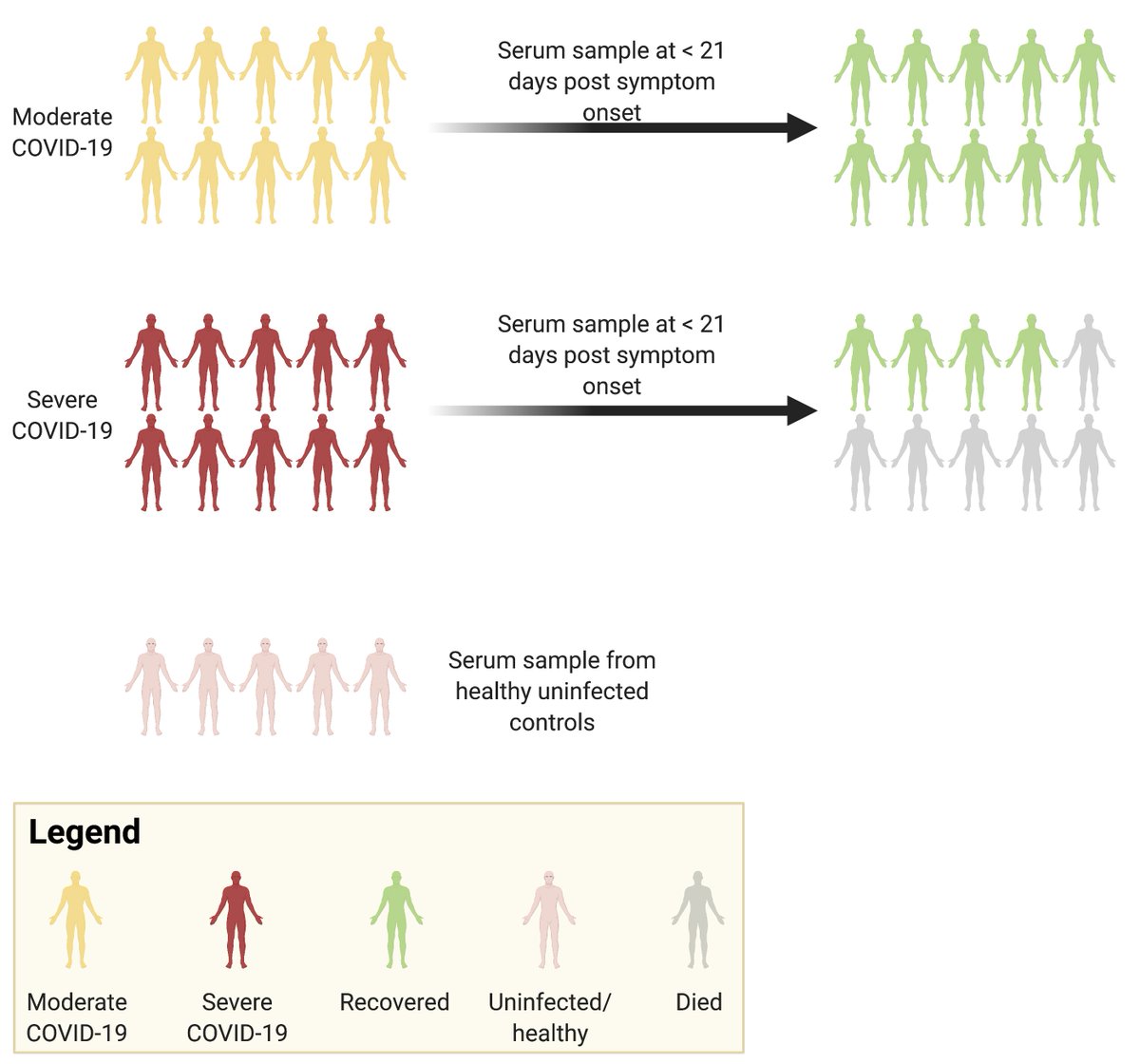
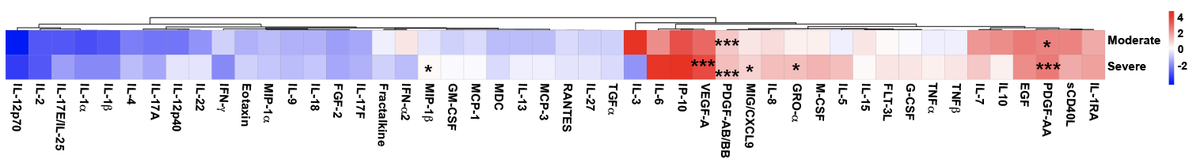
Consistent with other studies, we found that patients with moderate #COVID19 had lower serum levels of inflammatory cytokines and higher levels of #IFN-alpha, relative to healthy individuals. 

Consistent with other studies, we found that patients with severe #COVID19 had higher levels of inflammatory cytokines and lower levels of #IFN-alpha. 
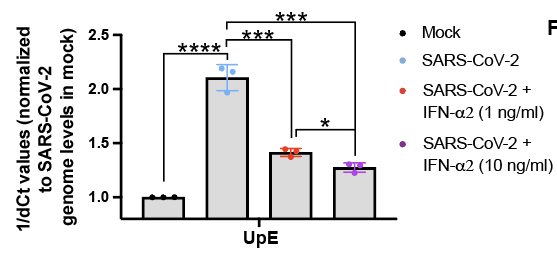
Importantly, we found that physiological levels of #IFN-alpha in patients with moderate #COVID19 was sufficient to reduce #SARSCoV2 replication in human airway cells. Thus, #IFN-alpha levels in these patients may be sufficient to restrict virus replication to some extent. 
Now, here is what we still do not know:
Studies have shown that #SARSCoV2 proteins, when over-expressed, can modulate #IFN responses. Some recent studies, including ours, show that wildtype virus infection turns on #IFN responses in human airway (Calu-3) cells. However..
Studies have shown that #SARSCoV2 proteins, when over-expressed, can modulate #IFN responses. Some recent studies, including ours, show that wildtype virus infection turns on #IFN responses in human airway (Calu-3) cells. However..
We do not yet know the extent to which SARSCoV2 proteins can block #IFN responses when expressed from a replicating virus. To answer this question, we would need to compare wildtype and ORF (protein) deletion variants of SARSCoV2 side-by-side. This is important.
Type of #IFNs and activation of #IFNs is cell-type dependent. The whole breadth of #SARSCoV2 – IFN interactions remains to be studied using a full range of permissive cell types.
A lot remains to be understood about how #coronaviruses, including #SARSCoV2 interact with different cellular players in the human body at different times post infection. Our study sheds light on some early events that occur in SARSCoV2 infected cells.
This was a huge interdisciplinary collaboration between multiple groups at @UofT, @McMasterU, @Harvard, @UWaterloo. Folks on twitter: @BaidKaushal @BatResearch, @jeremyhirota, @Millerlab_atMac, @BoWang87, @agmcarthur, @ACDoxey @MossmanLab
Thanks to funding agencies @NSERC_CRSNG @CIHR_IRSC @InnovationCA for supporting this work, along with support from various team members at @McMasterIIDR.
It took us a long time to get this story out. But we would like to thank the anonymous reviewers who helped and guided us through the way during an unprecedentedly busy time for virologists and immunologists. So, thank you!
We all know the importance of a well organized supply chain in the laboratory. @sue_science runs @MossmanLab flawlessly, supporting and facilitating all our cumulative work. Thanks to @NaderSayes too.
• • •
Missing some Tweet in this thread? You can try to
force a refresh