1/
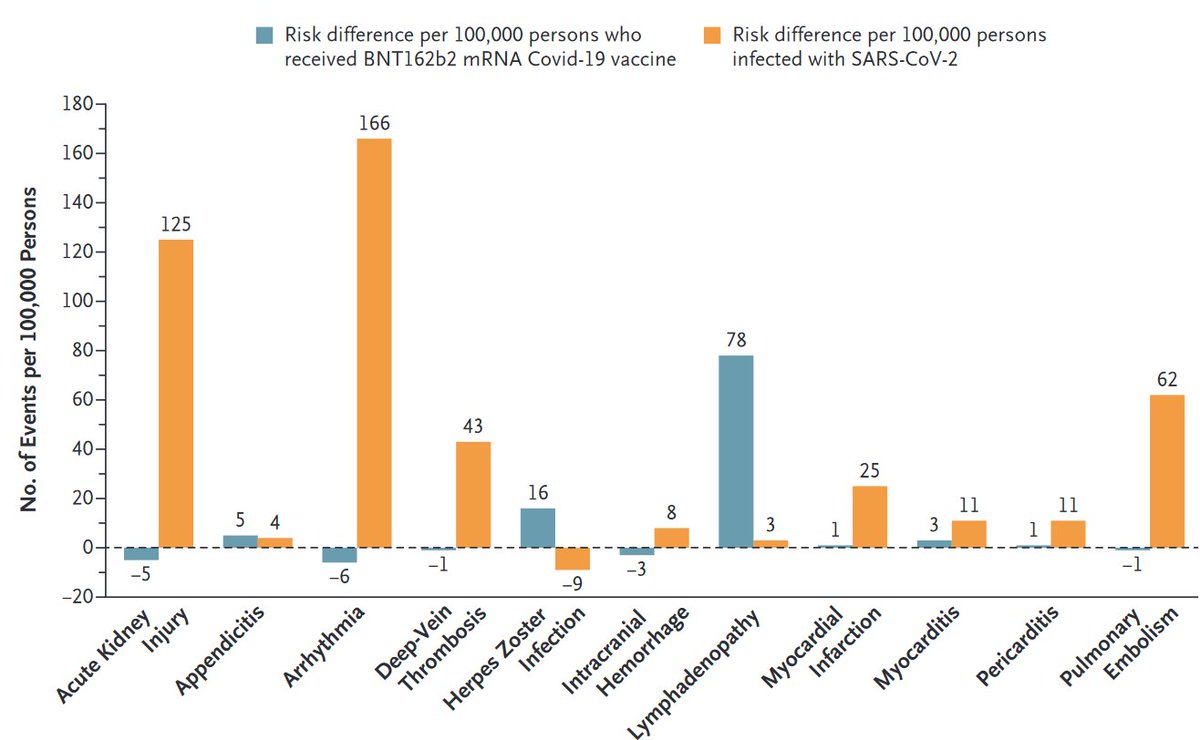
Vaccine safety: We compared excess adverse events after #COVID19 vaccination (Pfizer-BioNTech) and after documented #SARSCoV2 infection.
nejm.org/doi/full/10.10…
Take-home message: Low excess risk of adverse events after vaccination, higher after infection.
Some thoughts👇
Vaccine safety: We compared excess adverse events after #COVID19 vaccination (Pfizer-BioNTech) and after documented #SARSCoV2 infection.
nejm.org/doi/full/10.10…
Take-home message: Low excess risk of adverse events after vaccination, higher after infection.
Some thoughts👇
2/
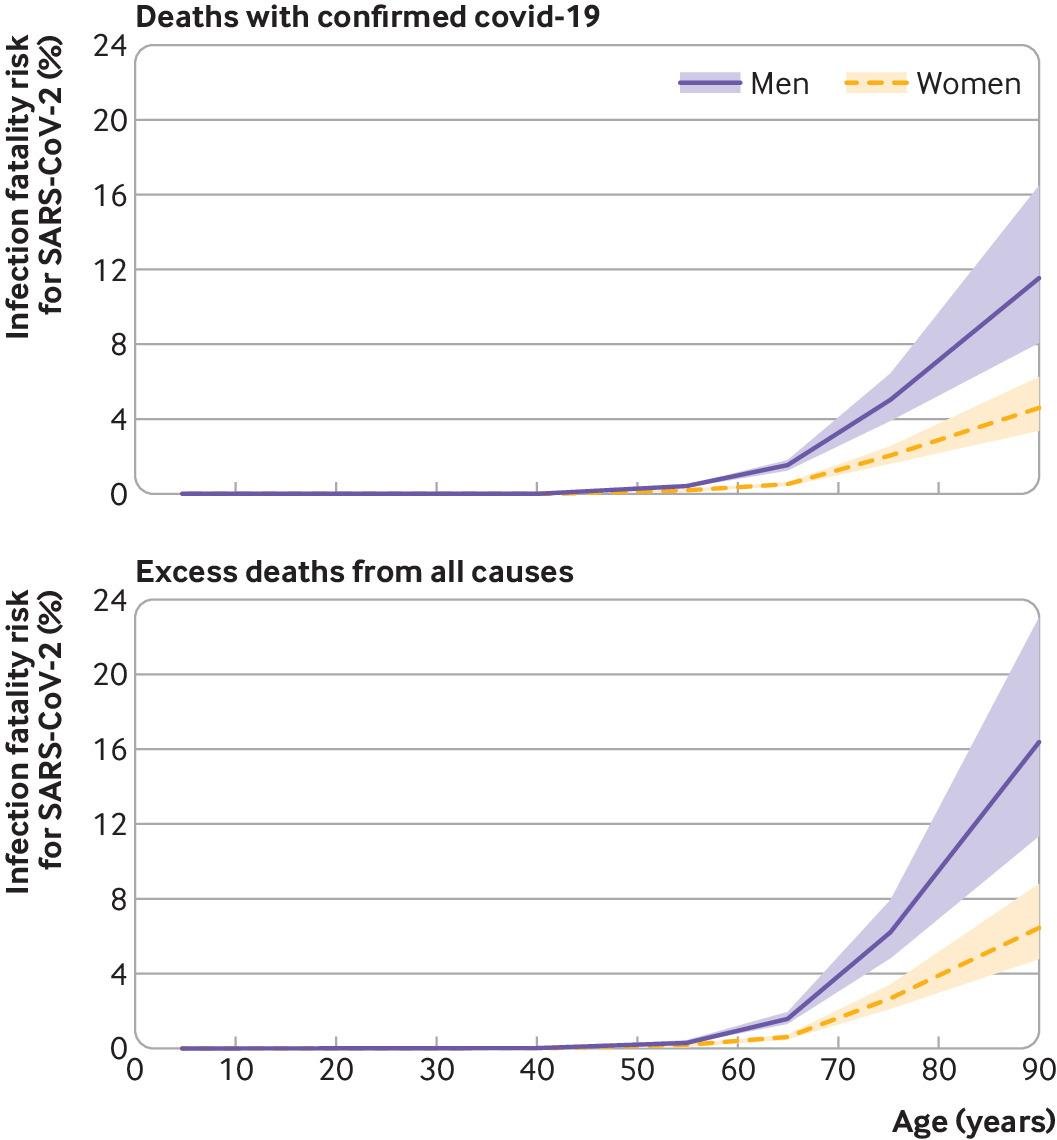
Preferring #SARSCoV2 infection over vaccination has become even harder. (Remember: infection also increases the risk of severe disease/death)
This is a good illustration of how #randomized trials and #observational studies complement each other for better #causalinference...
Preferring #SARSCoV2 infection over vaccination has become even harder. (Remember: infection also increases the risk of severe disease/death)
This is a good illustration of how #randomized trials and #observational studies complement each other for better #causalinference...
3/
The original #randomized trial estimated vaccine effectiveness to prevent symptomatic infection, but was too small to quantify vaccine safety.
That's what #observational studies do.
Now a different sort of question: Why could we do this study in the first place?
2 reasons.
The original #randomized trial estimated vaccine effectiveness to prevent symptomatic infection, but was too small to quantify vaccine safety.
That's what #observational studies do.
Now a different sort of question: Why could we do this study in the first place?
2 reasons.
4/ First, we had DATA from millions of people.
We worked with high-quality data from @ClalitHealth, a health services organization that covers >50% of the Israeli population.
In contrast, looks like many countries aren't taking investment in healthcare databases seriously.
We worked with high-quality data from @ClalitHealth, a health services organization that covers >50% of the Israeli population.
In contrast, looks like many countries aren't taking investment in healthcare databases seriously.
5/
Maintaining population health databases, available for immediate use in a public health crisis, is a matter of national security. How else can you make timely policy decisions?
Israel, UK (@Opensafely), and a few others are making great progress. How is everybody else doing?
Maintaining population health databases, available for immediate use in a public health crisis, is a matter of national security. How else can you make timely policy decisions?
Israel, UK (@Opensafely), and a few others are making great progress. How is everybody else doing?
6/
Countries/states with fragmented, unlinked, or not readily available databases will keep relying on others.
What happens if your questions are different? e.g., what's the safety of a different vaccine in a different population?
But good DATA weren't enough for our study...
Countries/states with fragmented, unlinked, or not readily available databases will keep relying on others.
What happens if your questions are different? e.g., what's the safety of a different vaccine in a different population?
But good DATA weren't enough for our study...
7/
Second, we had health data EXPERTS
This study was led by a team of epidemiologists @ClalitResearch, an institute specifically created to make Clalit's data useful.
Governments: This is a good model
(also used by non-governmental organizations like @KPDOR and @KPWaResearch).
Second, we had health data EXPERTS
This study was led by a team of epidemiologists @ClalitResearch, an institute specifically created to make Clalit's data useful.
Governments: This is a good model
(also used by non-governmental organizations like @KPDOR and @KPWaResearch).
8/
Governments: Together with your health data resource, create/support a research institute. Then encourage external collaborations.
For this study, Clalit's experts worked with external experts @CAUSALab, @CCDD_HSPH, @Bos_CHIP, @HarvardDBMI
Everybody wins with collaboration
Governments: Together with your health data resource, create/support a research institute. Then encourage external collaborations.
For this study, Clalit's experts worked with external experts @CAUSALab, @CCDD_HSPH, @Bos_CHIP, @HarvardDBMI
Everybody wins with collaboration
• • •
Missing some Tweet in this thread? You can try to
force a refresh