b/c there is no consensus on multiplicity issue: When, Why and How to deal with it?
-I’ll try to explain by using *frequentist* point of view
1/
2/
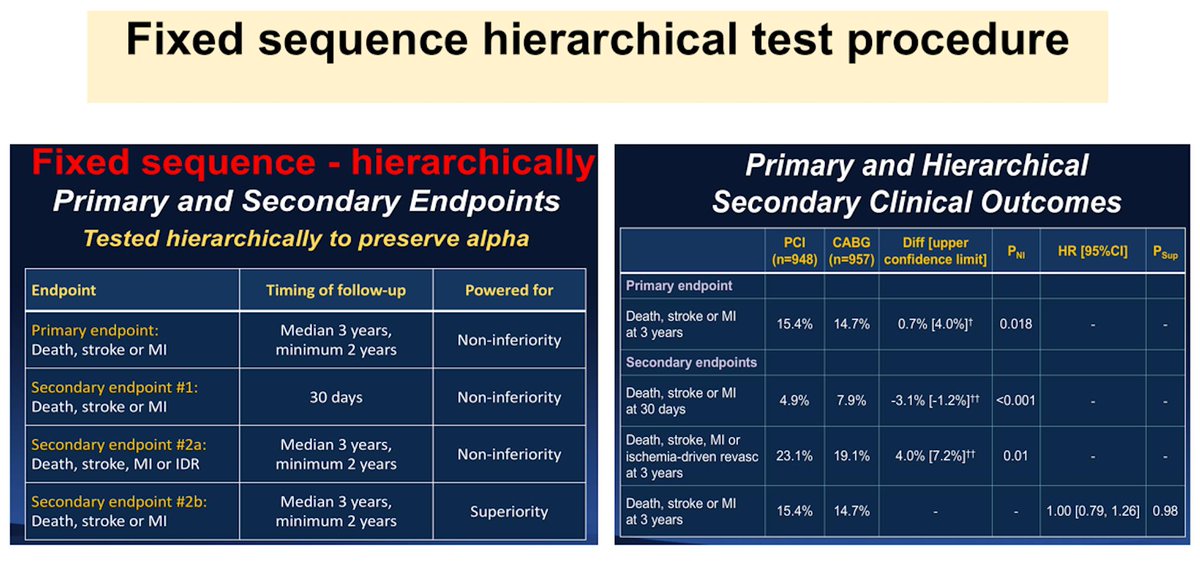
1-Multiple endpoints (i.e. death and death+HF rehosp)
2-Multiple treatments/arms (i.e. PCI vs Med-Tx vs CABG in pts with CAD or Riv15 mg vs Riv20 mg vs ASA)
3-Multiple interim analyses
4-Multiple subgroups
3/
*btw, to avoid loss of validity due to inflating Type I error from insufficient control, simultaneously avoiding power loss by excessive Type II error from overcontrol
5/
Some statisticians/trialists are againist it, however some advocate it.
6/
1-The simplest way is to avoid multiple comparison..
2-If multiplicity still persists
*Multiplicity adjustment should be considered
(depending on endpoints families, Tx arm, ......)
7/
10/
nonferroni >> qutoted from @stephensenn, @statsepi
13/
In the second step, the second largest p-value, 0.027, is compared to α/2 = 0.025; this p-value is also not statistically significant. Test then compares the next-largest p-value(0.020) and so on until the last p-value (0.010).
17/