This is an incredibly important preprint to inform #LongCovid. Among many analyses, the team recruited 4 patients w/ prolonged + recurrent olfactory function loss after #COVID-19 (time from first COVID-19 symptoms to inclusion ranged from 110-196 days): biorxiv.org/content/10.110… 
2/ None of these patients had detectable COVID-19 #RNA in nasopharyngeal samples by routine diagnosis (RT-qPCR). However, ALL patients had detectable COVID-19 RNA in samples obtained from their olfactory mucosa (confirmed with aRT-qPCR SYBR technique)
3/ Three of the patients had a high COVID-19 #viral load in the olfactory mucosa. Immunostaining additionally revealed the presence of COVID-19 antigens in 3 out of 4 patients. Based on that and related findings the team concluded...
4/ “...#SARS-CoV-2 has a significant tropism for the olfactory mucosa and, most importantly, we demonstrate that it can persist locally, not only a few weeks after general symptoms resolution but during several months in both mature and immature olfactory sensory #neurons.”
5/ In a further set of experiments, in addition to the olfactory bulb, #COVID-19 RNA was also detected in more remote #brain areas of infected hamsters, such as the cerebral cortex and the brainstem
6/ Leading the team to reference this separate Lancet study, which found COVID-19 viral RNA/protein in the brainstem of COVID-19 human patients via autopsy: thelancet.com/journals/laneu… 

7/ I want to further note that the overall trend (certain #pathogens may infect the brain via the nose/olfactory bulb is central to Harvard’s late Rob Moir + Rudy Tanzi’s model for CNS pathogen activity in Alzheimer’s: pubmed.ncbi.nlm.nih.gov/30314800/ 
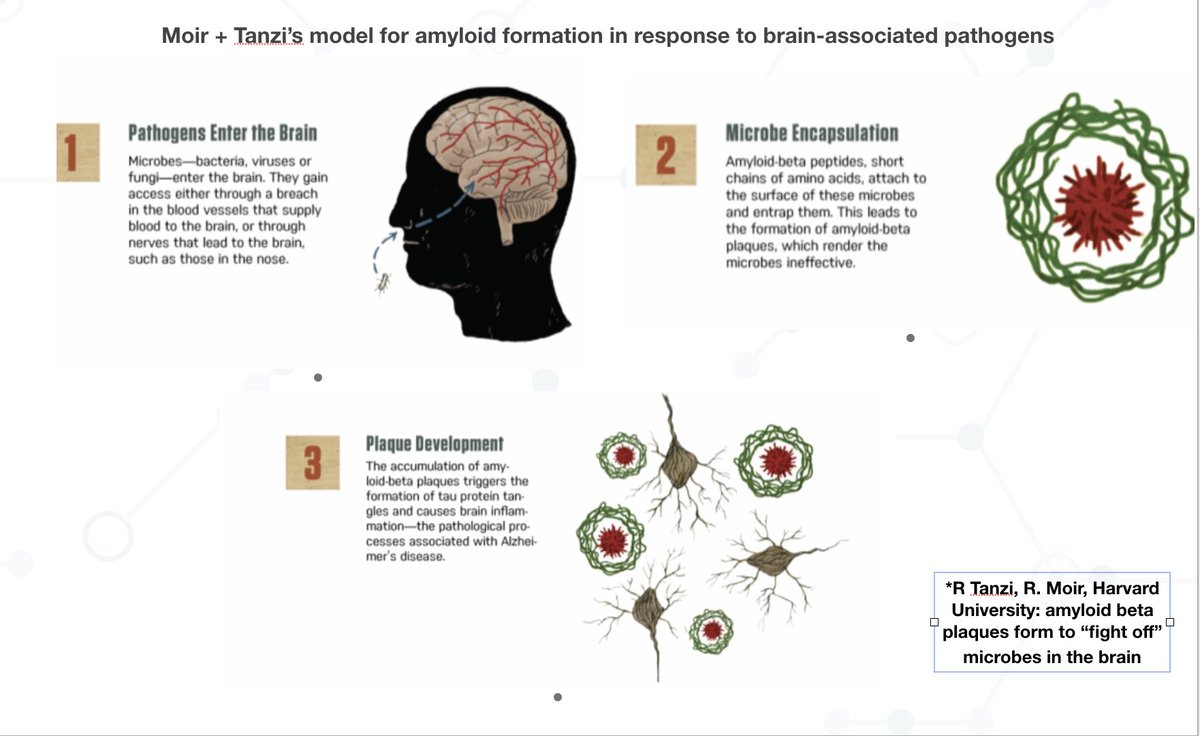
8/ Takeway: we are not going to understand #LongCovid (or #Alzheimer’s or #MECFS for that matter) unless we 1) analyze patient tissue/biopsies and use techniques that go far beyond routine testing 2) are open-minded to #pathogen entry + persistence in the central nervous system
• • •
Missing some Tweet in this thread? You can try to
force a refresh










