The chase for an #HIV cure has been elusive for decades with as many failures as seen with CNS diseases. $SGMO has been dedicated to this effort tho are now supporting sponsors given the cost of the effort. This stream will provide an recap. #Autologous #CellTherapy
1/
1/
$SGMO began this clinical effort in 2009. They have worked with UPenn, City of Hope, Case Western University. NAID and CIRM have helped with funding among others. We should see some critical updates this year.
#HIV
2/
#HIV
2/

The first trial (sponsored by UPenn) SB-728 began in 2009 focused on HAART treated patients and was focused on safety and comparison/persistence of CD4/CD8 T cells after ZFN modifed autologous T cells were infused.
#HIV #CellTherapy
3/
#HIV #CellTherapy
3/

$SGMO ran SB-728-0902 to find out whether #ZFN modified CD4+ T cells are safe and how these cells affect #HIV. #Autologous #CellTherapy
4/
4/

$SGMO ran a second trial SB-728-1002 beginning in 2010 which continued the effort of determining the effect of escalating #ZFN modified T cells post HAART interruption.
#HIV #Autologous #CellTherapy
5/
#HIV #Autologous #CellTherapy
5/

$SGMO continued to invest in this effort with the next trial SB-728-1101 in C11 which upped the dosage to 5-to-30b #ZFN modified T cells. This trial was to determine the impact of escalating doses of cyclophosphamide as a pretreatment regimen.
#HIV #Autologous #Celltherapy
6/
#HIV #Autologous #Celltherapy
6/

SB-728-mR-1401 was initiated by $SGMO in C14 to better understand treatment impact of 2 vs 3 doses of #ZFN modified T cells increasing the dosage up to 40b cells supported by safety data from earlier trials.
7/
7/

It is pretty remarkable how much $$$ $SGMO invested in this effort even tho they knew how many years the process would take given the step function of the trials to date. In 2015 UPenn with funding from NIAID sponsored the first trial focused on SB-728mR. Note conclusion
8/
8/

The City of Hope with Paula Cannon and CIRM funding sponsored SB-728mR-HSPC. This trial had an estimated primary completion of last month. It includes the impact of busulfan levels with the #ZFN edited T cells. Safety and dosing have led to treatment w/preconditioning.
#HIV
9/
#HIV
9/

Case (Sekaly, Rodriguez) began their next trial in Jun 2019 focusing on replication-competent HIV reservoir. This trial is still recruiting. 2 yrs of data are required before primary completion date of Jan 2024. #ZFN $SGMO
#HIV #Trailblazer #Reservoir
10/
#HIV #Trailblazer #Reservoir
10/

Pablo Tegas is the investigator in the next UPenn sponsored trial that was initiated in Jul 2019. This trial compares treatment interruption after $SGMO #ZFN infusion. The primary completion data is at the end of this year.
11/
11/

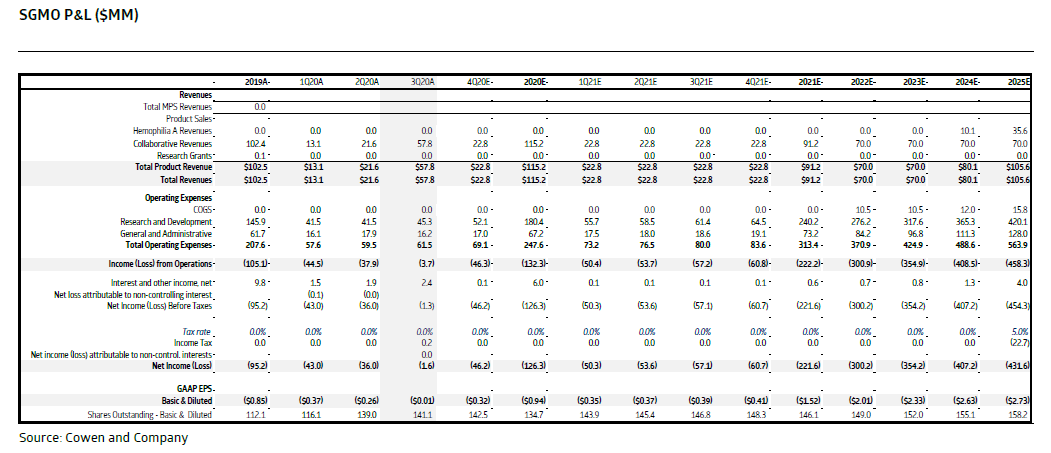
$SGMO is also running a LT followup study for patients treated with SB-728-T or SB-728mR cells. I found the Oct 2020 Cowen report interesting as they have concluded that the cum data provides POC for the #CellTherapy platform technology.
12/


12/


@threadreaderapp unroll please
• • •
Missing some Tweet in this thread? You can try to
force a refresh