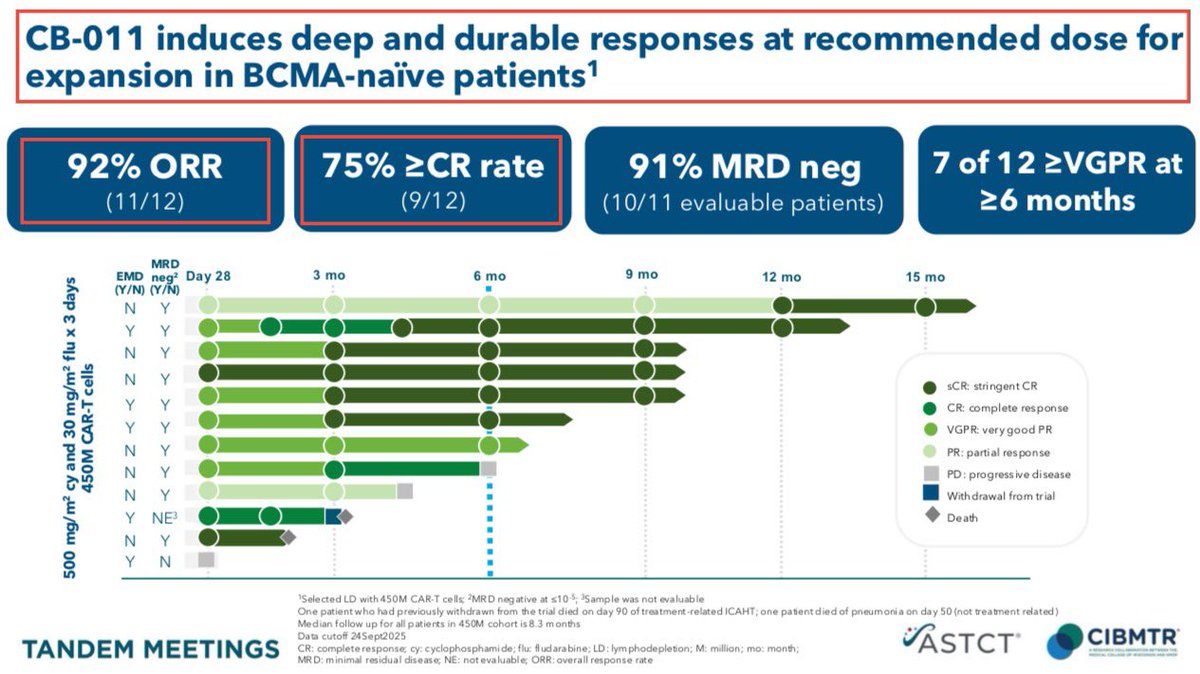
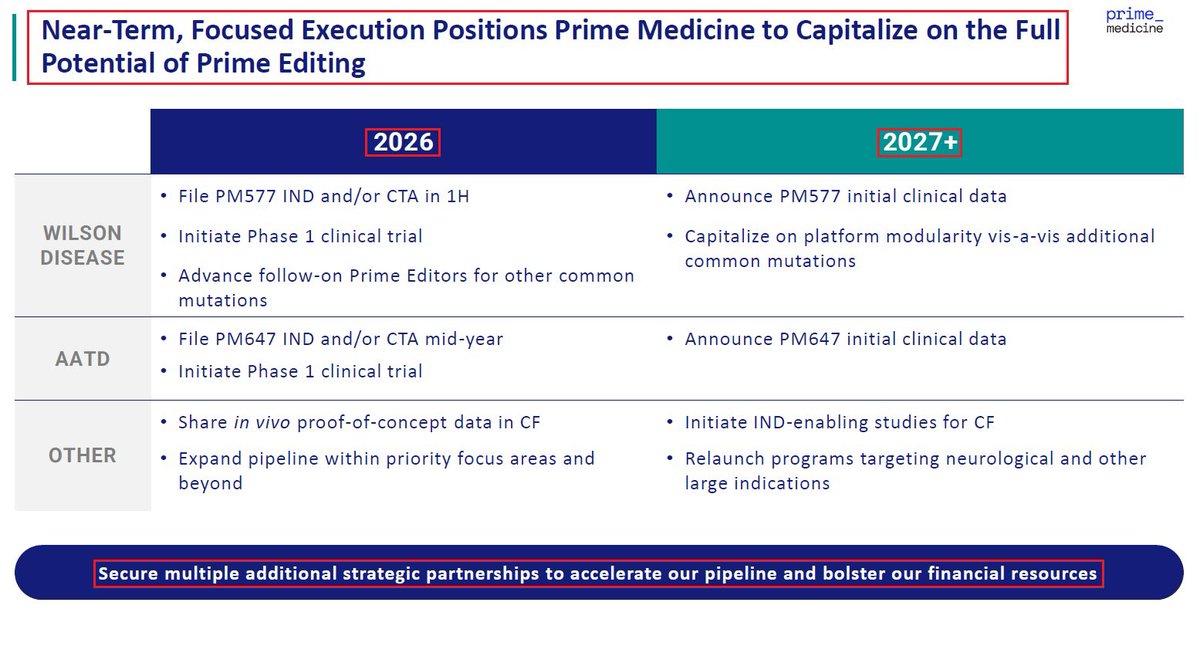
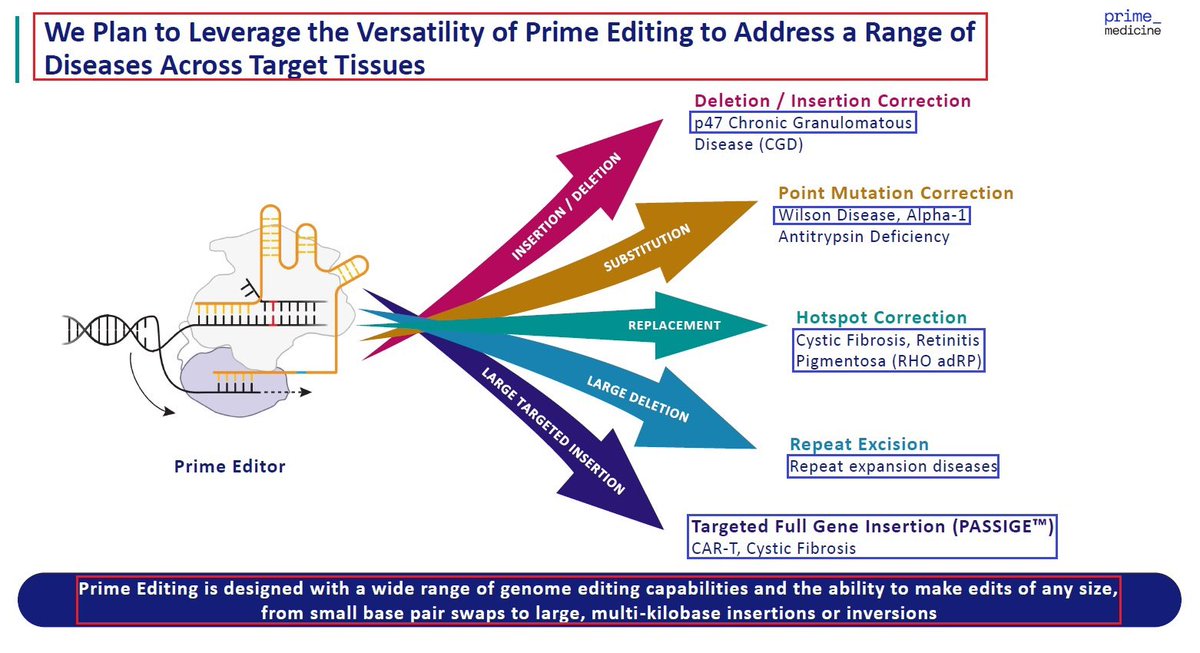
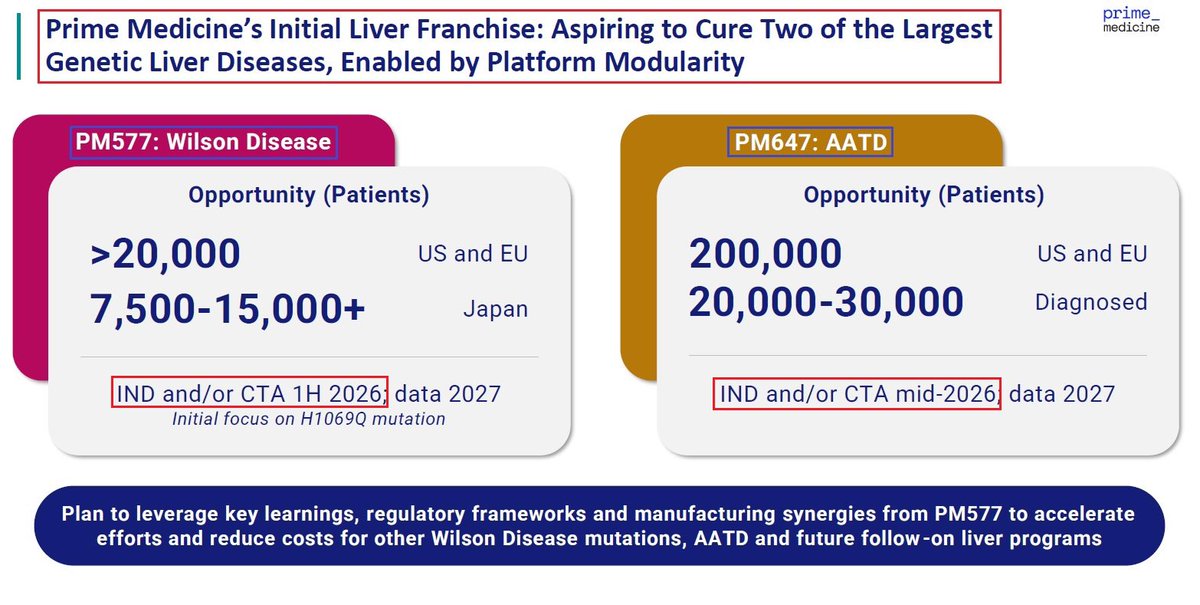
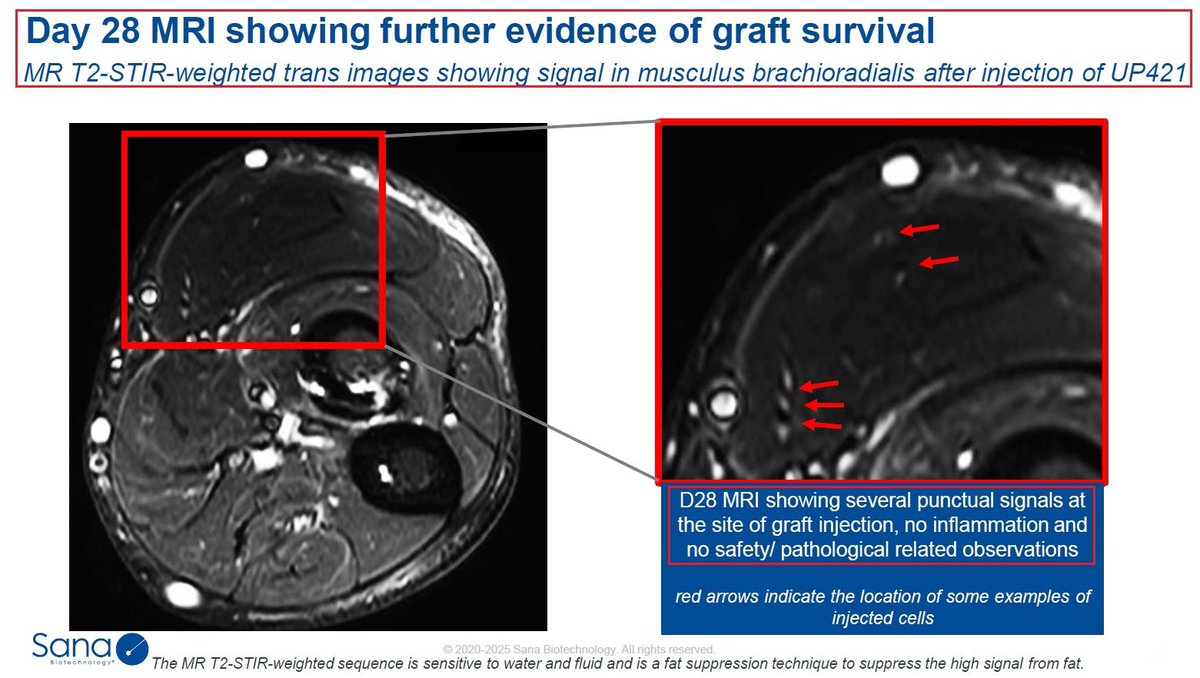
1/@GraphiteBio presented preclinical results supporting the use of a single-#cell #RNA #sequencing method to assess #gene correction outcomes in #patients treated with nulabeglogene autogedtemcel (nula-cel) - GPH-101. #BioTech #CRISPR #GeneEditing #Genomics $GRPH #ASH22 
2/GPH101 (nula-cel) is a #CRISPR #Cas9 #GeneEditing autologous #stem #cell-based #therapy in clinical development aimed to treat #SickleCellDisease. GPH101 is designed to directly correct the underlying mutation, thereby decreasing HbS production & restoring HbA expression #ASH22 

3 $GRPH gene correction platform involves editing hematopoietic #stem cells found in the #bonemarrow that develop into various types of #blood #cells. Since red blood cells lose their genomic DNA during maturation-tracking #GeneEditing in mature cells via sequencing is impossible 

4/The problem of the inability to mesure the efficacy of #GeneEditing in #SCD patients was solved by using immature red blood cells called #reticulocytes which retain their RNA and can be sequenced in order to assess the #gene correction levels after using nula-cel. #ASH22 
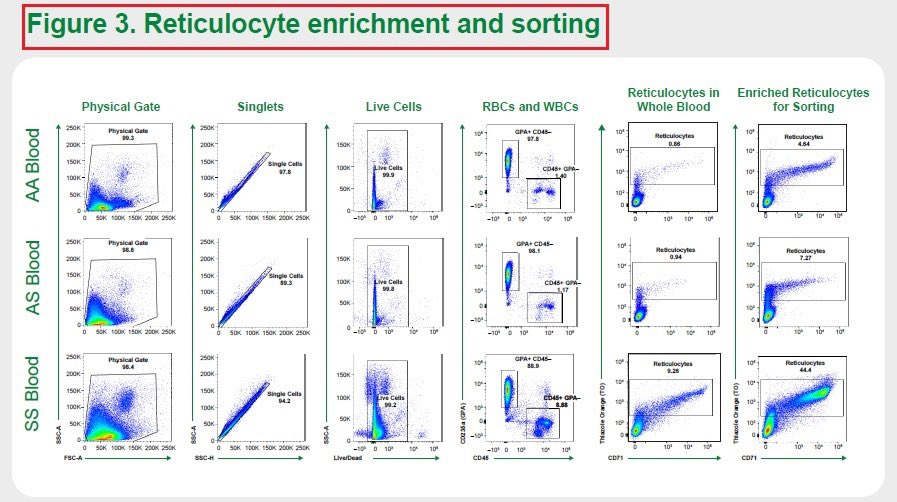
5/Based on this knowledge, $GRPH sought to develop a single-cell RNA sequencing method that could measure #GeneEditing outcomes in reticulocytes. It first measured the genetic makeup of reticulocytes from healthy donors-AA, people with SCD trait-AS, & with #sicklecelldisease-SS. 

6/Results from both experiments demonstrated the single-cell RNA sequencing method’s ability to precisely and reproducibly measure and differentiate the healthy donors - AA, people with SCD trait - AS and #SickleCellDisease patients - SS. #ASH22 
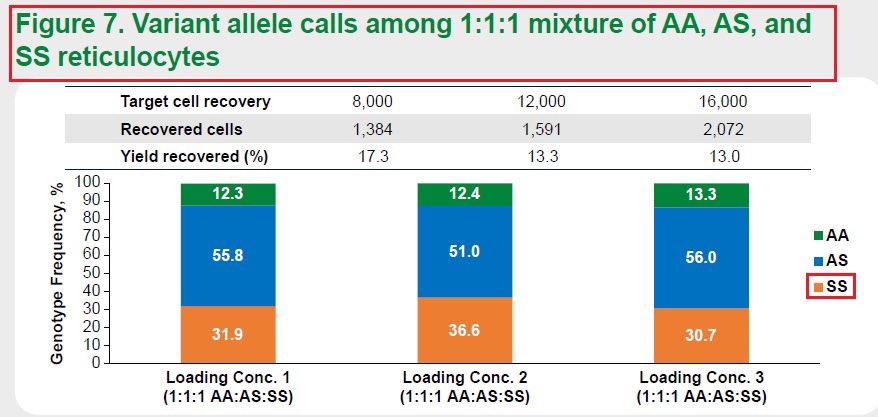
7/These data support the use of Single-cell #RNA sequencing of #reticulocytes to determine initial #GeneEditing outcomes in #SickleCellDisease patients treated with nula-cel (GPH101) thus supporting the clinical development of @GraphiteBio’s investigational #therapy. #ASH22 

• • •
Missing some Tweet in this thread? You can try to
force a refresh