A 🧵about 🧠problems after C19 💉
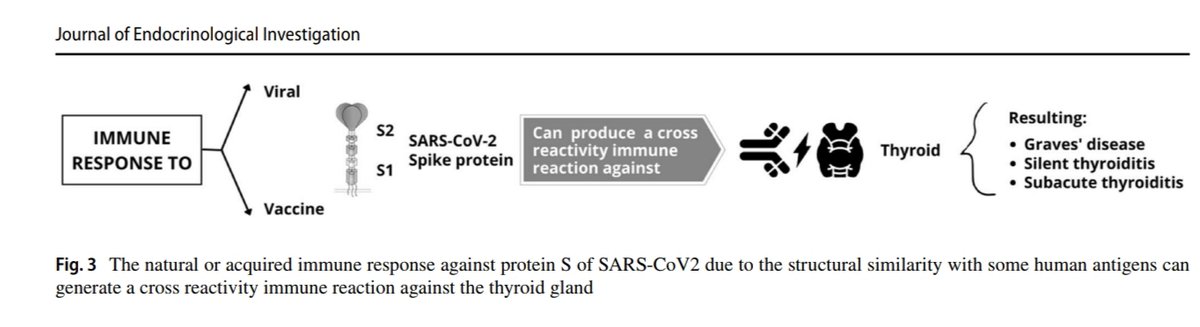
1. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection.
“There was an increased risk of Guillain–Barré syndrome and Bell’s palsy with ChAdOx1nCoV-19, hemorrhagic stroke with BNT162b2. ..An independent
1. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection.
“There was an increased risk of Guillain–Barré syndrome and Bell’s palsy with ChAdOx1nCoV-19, hemorrhagic stroke with BNT162b2. ..An independent
Scottish cohort provided further support for the association between ChAdOx1nCoV and Guillain–Barré syndrome, there was a substantially higher risk of all neurological outcomes in the 28 days after a positive SARS-CoV-2 test including Guillain–Barré syndrome. Overall, we
estimated 38 excess cases of Guillain–Barré syndrome per 10 million people receiving ChAdOx1nCoV-19 and 145 excess cases per 10 million people after a positive SARS-CoV-2 test.” 


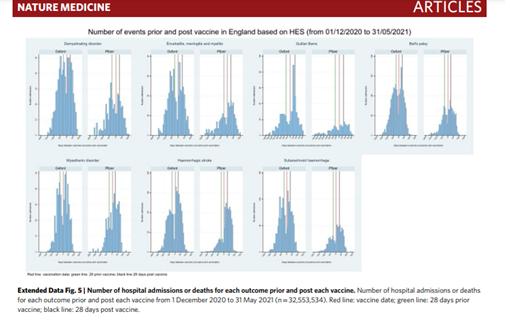
2. “As of June 29, 2021, among the 1,257,497 cases reported with COVID-19 vaccines, 1,256 (0.1%) “acute polyneuropathies” (all cases corresponding to GBS and GBS variants) were reported (422 USA, 387 UK, 328 Europe [40 France], and 119 elsewhere). Among these, patients
with FP-GBS were identified using the MedDRA Preferred Terms: “Bell's palsy”, “facial paralysis”, “facial nerve disorder”, “facial paresis”, and “oculofacial paralysis”. A total of 142 of 1,256 GBS patients experienced FP (11.3%). This included 26 of 488 (5.3%) GBS patients who
received mRNA vaccines (12/328 [3.7%] Pfizer–BioNTech, 14/160 [8.8%] Moderna), 114 of 744 (15.3%) who received adenovirus-vectored vaccines (86/630 [13.7%] Oxford–AstraZeneca, 28/114 [24.6%] Johnson & Johnson), and 2 of 24 (8.3%) who received other vaccines.
FP-GBS was significantly more frequent after adenovirus-vectored vaccines.”onlinelibrary.wiley.com/doi/10.1002/an… 

3. Reply to ‘Adenovirus COVID-19 Vaccines and Guillain-Barré Syndrome with Facial Paralysis’ by Dr Pegat et al.
“This robust analysis indeed implies a higher association of facial paralysis with post vaccination Guillain-Barré Syndrome (GBS).
“This robust analysis indeed implies a higher association of facial paralysis with post vaccination Guillain-Barré Syndrome (GBS).
Although retrospective analysis of a pharmacovigilance database is likely to lead to bias and underreporting, the difference in rates of facial paralysis between adenovector vaccines and the mRNA vaccines is indeed striking.
Our series also noted a similar increase in bifacial paralysis with GBS after ChAdOX1 vaccination with 7/7 (100%) of our cases developing this clinical finding.”
onlinelibrary.wiley.com/doi/10.1002/an…
onlinelibrary.wiley.com/doi/10.1002/an…
4. "After excluding one classical case of acute motor axonal neuropathy following Campylobacter jejuni gastroenteritis, we calculated population GBS observed rates and compared these to historical background rates. The observed GBS incidence rate was
1.0 reports per 100,000 doses of ChAdOx1-S vaccine, higher than the expected background rate of 0.61 presentations per 100,000 adult population within 42 days of vaccination.
While temporal associations do not imply causality and spontaneous surveillance systems have limitations
While temporal associations do not imply causality and spontaneous surveillance systems have limitations
in capturing all adverse events following immunization, the observed disproportionality of vaccines involved was unexpected, with zero reports to SAEFVIC of GBS after the BNT162b2 mRNA vaccine. We do note there are also now case reports of GBS after SARS-CoV-2 mRNA vaccines.
Notwithstanding small numbers limiting further interpretation, our data demonstrate an excess of observed cases compared with expected, and disproportionate excess reporting of GBS following ChAdOx1-S vaccine."onlinelibrary.wiley.com/doi/full/10.10… 

5. Headache is by far the most frequent neurological side effect of SARS-CoV-2 vaccinations and occurs with any of the approved vaccines. In the majority of cases, headache starts within a few hours after the vaccination and resolves spontaneously within 48 h. However, a subacute
type of headache has been delineated which occurs on the average 8 days after the shot and is frequently associated with VST. The cause of headache after SARS-CoV-2 vaccinations remains speculative but generally, it can be tension-type headache due to stress, due to intracerebral
bleeding (ICB) or subarachnoid bleeding (SAB), due to vasospasms like in SAB or reversible, cerebral vasoconstriction syndrome (RCVS), or due to VST. As VST is frequently associated with ischemic stroke, ICB, or SAB, headache may be multi-causal in patients experiencing VST.
Thunderclap headache typically occurs with SAB or RCVS.
In conclusion, this study shows that safety concerns against SARS-CoV-2 vaccines are backed by an increasing number of studies reporting neurological side effects.
In conclusion, this study shows that safety concerns against SARS-CoV-2 vaccines are backed by an increasing number of studies reporting neurological side effects.

The most frequent of them are headache, GBS, VST, and transverse myelitis."onlinelibrary.wiley.com/doi/10.1111/an…
Not all the neurological side effects bcoz of the 💉 are reported, not bcoz the numbers are few. No reports from another brand, doesn't mean no issues. #Uncovered issues.
end.
#raise the awareness of #CovidVaccine #sideeffects
end.
#raise the awareness of #CovidVaccine #sideeffects
• • •
Missing some Tweet in this thread? You can try to
force a refresh