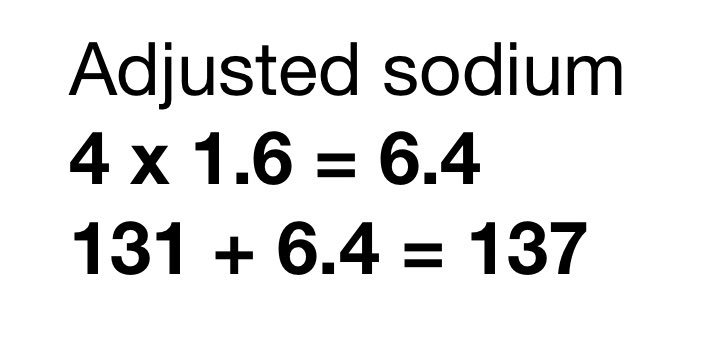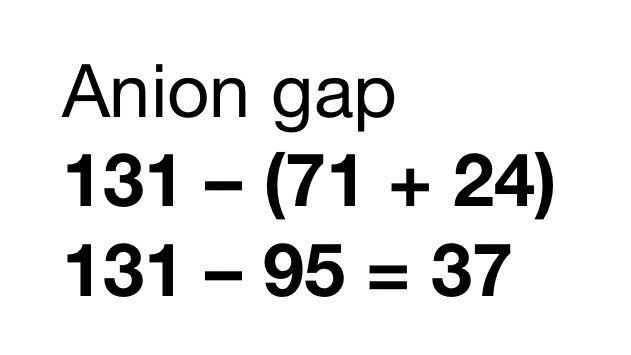THE METABOLIC SEESAW. YEE HAW.
1/12
dBC dAG
———————🔺———————
(more BC)
dAG = delta Anion Gap
dBC = delta Bicarbonate.
Toward the Center = more bicarbonate
This will all make sense in a moment.
2/12
Your ?: is their compensation appropriate? Do I need more labs? Who and what is a winter and why do they have a formula?!?!
Answers: seesaw only, a chemistry is all you need!
3/12
dBC: 22 - lab bicarb
Lab = on your chem panel.
The dAG can be thought of as the increase in acid. Which means, you’d expect an equal decrease in bicarbonate! How much did the bicarb decrease? That’s the dBicarbonate!
So, why seesaw? EZ to see 😉
4/12
This makes sense since the dAG = acid rise, and dBC = base fall. More acid = less base. These should balance each other out if there is no other process happening!
5/12
AG: 20, Bicarb: 14
dAG: 20-12 = 8
dBC: 22-14 = 8
dBC dAG
———————🔺———————
(8 from 🔺) (8 from 🔺)
There is balance in the force, this is a pure anion gap metabolic acidosis.
6/12
dAG: 25-12 = 13
dBC: 22-20 = 2
dBC dAG
———————🔺———————
( 2 from 🔺) ( 13 from 🔺)
IMBALANCE: dBC wants to be 13 out like dAG is but something is pushing it up!
7/12
Next example:
8/12
dAG: 20-12 = 8
dBC: 22-3 = 19
dBC dAG
———————🔺———————
( 19 from 🔺) ( 8 from 🔺)
IMBALANCE: dBC wants to be 8 out like dAG is but something is pushing it further down! Something is pushing DOWN the bicarbonate!
9/12
10/12
1. Draw your see-saw (dAG on either side)
2. Remember that the dBC wants to balance it
3. Ask, is dBC being pushed away from center by where it should be if it was balanced, or toward center by where it should be if it was balanced?
11/12
Happy seesawing!
12/12
#medtwitter
#medstudenttwitter
#FOAMed #FOAM