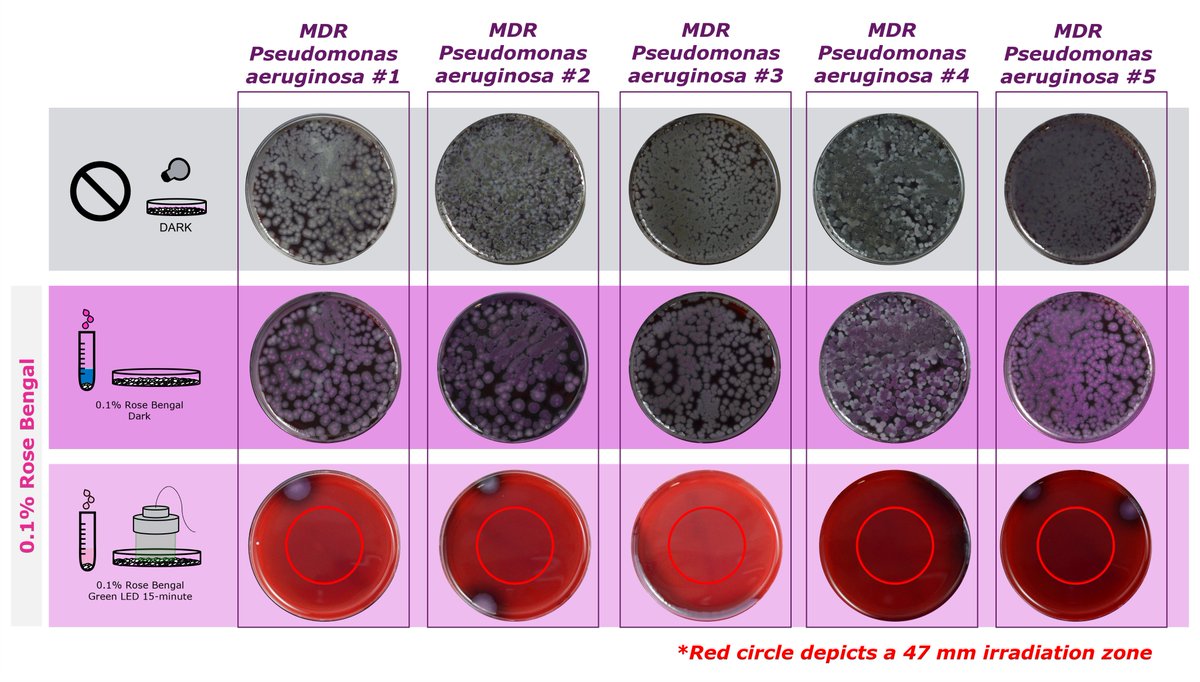
1/ A $PVCT THREAD: (3) Potential 2023 read outs of preclinical data from sponsored research programs (#SRAs) in different diseases. #research. #oncology #hematology #dermatology #InfectiousDiseases #ophthalmology #WoundHealing #TissueRegeneration. #rosebengal #rosebengalsodium.
2/ $PVCT 2023 Stockholder Letter (released January 9th).
https://twitter.com/ProvectusBio/status/1612438924206702594. #rosebengal #rosebengalsodium.
3/ In 2023, $PVCT expects research collaborators at different academic medical institutions to read out data from their preclinical research work under newly established and existing #SRAs. #research. #rosebengal #rosebengalsodium.
4a/ From $PVCT’s founding (2002) sec.gov/Archives/edgar…, preclinical research was done primarily, if not exclusively, in-house. Many firms do this in the major US biotech hubs of #Boston, #SanFrancisco, #SanDiego, #ResearchTrianglePark, etc.
4b/ Key published $PVCT work included (2002) “Functional Imaging of Photosensitizers using Multiphoton Microscopy” provectusbio.com/media/docs/pub….
4c/ $PVCT (2003): “Topical Rose Bengal: Pre-Clinical Evaluation of Pharmacokinetics and Safety” provectusbio.com/media/docs/pub….
4d/ Extramural research was limited and involved $PVCT (2006): “Rose Bengal induces dual modes of cell death in melanoma cells and has clinical activity against melanoma” provectusbio.com/media/docs/pub….
4e/ $PVCT alone: Society for Immunotherapy of Cancer (SITC) 2012: “Generation of an Antitumor Response and Immunity Using a Small Molecule Drug (PV-10)” provectusbio.com/media/docs/pub….
4f/ $PVCT alone: American Association for Cancer Research (AACR) 2013: “Combination of PV-10 Immuno-chemoablation and Systemic anti-CTLA-4 Antibody Therapy in Murine Models of Melanoma” provectusbio.com/media/docs/pub….
5/ Limited or no outside validation, such as third-party medical academic researchers, medical conference presentations, and peer-reviewed medical journal articles, may lead to more questions than answers – if you’re a biotech industry outsider with no recognized pedigree.
6a/ $PVCT’s first key research collaboration began in 2011 with Moffitt Cancer Center in Tampa, FL @MoffittResearch. @spt_lab became a key #TranslationalResearcher labpages2.moffitt.org/pilon-thomas/. #research. #rosebengal #rosebengalsodium.
6b/ The initial goals of @MoffittResearch’s @spt_lab’s #SRAs were to validate PV-10’s mechanism of action (MOA) and elucidate its mechanism of immune action (MOIA). #research. #rosebengal #rosebengalsodium.
6c/ @spt_lab MOA: 1st medical presentation at Society of Surgical Oncology (SSO) 2012, “Intralesional Injection of Melanoma with Rose Bengal Induces Regression of Untreated Synchronous Melanoma In a Murine Model” provectusbio.com/media/docs/pub….
6d/ @spt_lab MOIA: 1st journal article (2013) @PLOSONE: Intralesional Injection of Rose Bengal Induces a Systemic Tumor-Specific Immune Response in Murine Models of Melanoma and Breast Cancer journals.plos.org/plosone/articl….
6f/ @spt_lab eventually did even more consequential work. $PVCT PV-10 + checkpoint: “Efficacy of Intralesional Injection with PV-10 in Combination with Co-Inhibitory Blockade in a Murine Model of Melanoma” at SITC 2014 provectusbio.com/media/docs/pub….
6g/ @spt_lab’s SITC 2014 PV-10 + checkpoint combination work validated $PVCT’s AACR 2013 work.
6h/ $PVCT. more @spt_lab: Immune system activation, signaling, and response (2016): “Intralesional rose bengal in melanoma elicits tumor immunity via activation of dendritic cells by the release of high mobility group box 1” oncotarget.com/article/9247/t….
6i/ $PVCT. more @spt_lab: Immune mechanism of immune combination therapy (2018): “T cell mediated immunity after combination therapy with intralesional PV-10 and blockade of the PD-1/PD-L1 pathway in a murine melanoma model” journals.plos.org/plosone/articl….
6j/ $PVCT. more @spt_lab: PV-10 + chemo (2021): “Intralesional injection of rose bengal augments the efficacy of gemcitabine chemotherapy against pancreatic tumors” bmccancer.biomedcentral.com/articles/10.11….
7a/ A 2nd $PVCT collaboration began in ~2014/15 with the University of Illinois at Chicago @thisisUIC and another key #researcher @Maker_MD chicago.medicine.uic.edu/ajay-maker-lab/. #rosebengal #rosebengalsodium.
7b/ $PVCT @Maker_MD MOA: 1st poster presentation at SSO 2015: “Intralesional Injection of Rose Bengal Induces an Anti-tumor Immune Response and Potent Tumor Regressions in a Murine Model of Colon Cancer” expo.jspargo.com/exhibitor/web/…. IMAGES. 



7c/ $PVCT @Maker_MD MOIA: 1st journal article (2017): “Colon cancer cell treatment with rose bengal generates a protective immune response via immunogenic cell death” provectusbio.com/media/docs/pub….
8/ @spt_lab @Maker_MD. 3rd-party, extramural, arm’s length, sponsored scientific research that separately (a) reproduced $PVCT’s PV-10’s cytotoxic effect on cancer cells (MOA), (b) elucidated PV-10’s MOIA, and (c) did in different types of cancers: #melanoma, #coloncancer.
9/ Historical context, $PVCT had completed Phase 1 & 2 trials in #melanoma, and treated a number of #livercancer patients in a Phase 1 study, collecting both safety and activity/efficacy data, prior to @spt_lab’s and @Maker_MD’s MOA and MOIA work.
10a/ Interestingly, unaffiliated Italian researchers validated $PVCT’s PV-10’s MOA and began identifying PV-10’s MOIA (when they believed that light activation was required) before @spt_lab and @Maker_MD.
10b/ Full Professor Luciana Dini of Sapienza University of Rome. Associate Professor Elisa Panzarini of the University of Salento.
10c/ 2010: “Rose bengal acetate photodynamic therapy-induced autophagy” pubmed.ncbi.nlm.nih.gov/20935508/.
10d/ 2014: “Rose Bengal acetate photodynamic therapy (RBAc-PDT) induces exposure and release of Damage-Associated Molecular Patterns (DAMPs) in human HeLa cells” pubmed.ncbi.nlm.nih.gov/25140900/.
10e/ $PVCT Comfortingly, PV-10 MOA and MOIA with light = PV-10 MOA and MOIA without light.
11a/ More historical context, a group of stockholders (PRH) entered into a Definitive Financing with $PVCT in 2017. prnewswire.com/news-releases/….
11b/ The reproducibility of $PVCT medical science, both under sponsored and in unaffiliated study, made due diligence in this area straightforward during the chaotic time of negotiating the 2017 Definitive Financing.
12/a Reproducibility is the hallmark of science. “This is one of medicine's dirty secrets: Most results, including those that appear in top-flight peer-reviewed journals, can't be reproduced” (2011) wsj.com/articles/SB100….
12b/ “Is science suffering from a reproducibility crisis? How do we really know what we know?” (2015) statnews.com/2015/12/03/pod….
13a/ Since 2017, $PVCT has been undertaking basic medical, translational, and clinical research in a distributed-network and cross-disease manner. #research. #rosebengal #rosebengalsodium.
13b/ Different researchers and clinicians pursue different medical scientific hypotheses of $PVCT’s RBS’ therapeutic potential in different disease areas based on their area of expertise and in consultation with us.
13c/ $PVCT emphasizes the observations and conclusions of one researcher, and our knowledge and prior experience, in one disease area with other researchers in other disease areas.
13d/ Furthermore, $PVCT encourages collaboration between our various researchers to assist us in building our medical science platform and associated drug pipeline.
13e/ $PVCT ultimately let #rosebengalsodium out of its box to thrive, or shrivel, in more and different settings.
14a/ $PVCT has 4 existing research collaborations.
14b/ Moffitt Cancer Center in Tampa, Florida (oncology).
14c/ The Rockefeller University in New York, New York (dermatology).
14d/ The Cumming School of Medicine at the University of Calgary in Alberta, Canada (oncology, hematology, vaccines, and infectious diseases).
14e/ The University of Tennessee Health Science Center in Memphis (infectious diseases).
15a/ $PVCT established 4 new research collaborations in 2022.
15b/ The College of Veterinary Medicine at the University of Tennessee in Knoxville (animal health).
15c/ Bascom Palmer Eye Institute at the University of Miami in Florida (ophthalmology).
15d/ The University of Texas Medical Branch in Galveston (wound healing).
15e/ The University of Nevada, Las Vegas (tissue regeneration and repair).
16a/ $PVCT’s work to oversee and facilitate the regular connectivity of this distributed research network endeavors to affirm that outcomes are, or are not, mechanistically consistent.
16b/ $PVCT’s collaborators and we are working to show that RBS’ bifunctional disease targeting (direct contact between RBS & disease leading to cell death/protein repair; such contact then catalyzing multivariate immune signaling, activation & response) is, or not, consistent.
17a/ $PVCT’s 2023 stockholder letter (issued January 9th) ➧ globenewswire.com/news-release/2….
17b/ $PVCT ➧ Focus (1): We plan to pursue a regulatory pathway for rare disease in-transit melanoma (a distinct sub-population of Stage III cutaneous melanoma) with monotherapy, intratumoral, small molecule, cancer immunotherapy PV-10®.
17c/ $PVCT ➧ Focus (2): We plan to design, prepare, and potentially commence a Phase 2/3 RCT of PV-10 + SOC checkpoint vs SOC checkpoint for 1st-line Stage III melanoma.
17d/ Regulatory/RCT clinical in oncology (melanoma, to start) are $PVCT’s priorities, while also seeking to demonstrate our potentially expansive halogenated xanthene (#rosebengalsodium) medical science platform for other diseases.
17e/ $PVCT ➧ Focus (3): We plan to receive readouts of ongoing and new preclinical research. #research. #rosebengal #rosebengalsodium.
17f/ An additional $PVCT clinical pursuit is metastatic #uvealmelanoma. Disease that is injectable via the liver (i.e., hepatic mets). NCT00986661 #livercancer. The initial work was done under the study’s Expansion Cohort 1: PV-10 +/- checkpoint(s). e.g., provectusbio.com/media/docs/pub….
17g/ $PVCT’s preparation of follow-on work for hepatic #uvealmelanoma mets is ongoing; would be under NCT00986661 EC3: PV-10 + checkpoint(s). Our data analysis and assessment were not complete prior to the 2023 stockholder letter, and not ready to communicate yet. THREAD END.
@threadreaderapp unroll
• • •
Missing some Tweet in this thread? You can try to
force a refresh