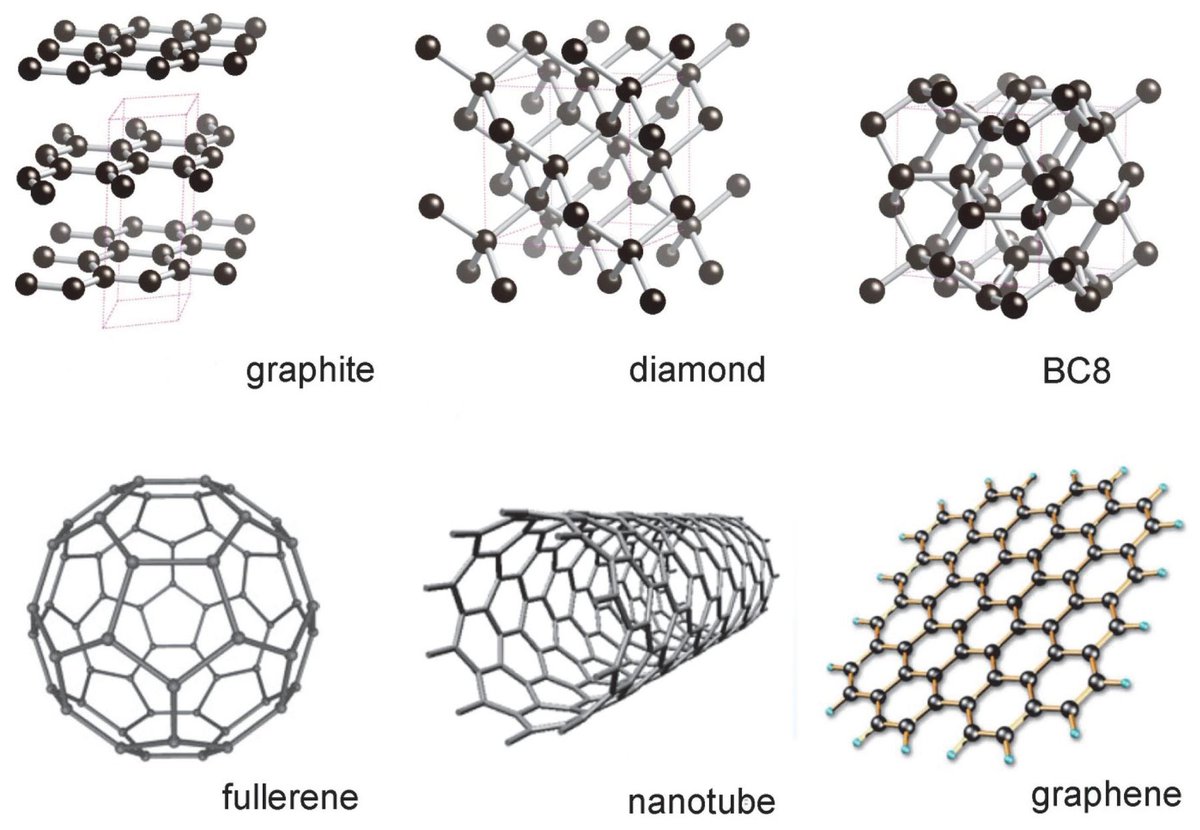
Crystals are minerals growing in specific HIGHLY ordered structures.
Crystals can be natural or synthetic.
Crystals can make me happy ('cause they're neat!)
Crystals are never magic.
Note: opalite is always synthetic.
For all my griping about not wanting to memorize All The Things for exams, mineralogy & crystallography are badass and deserve more respect than getting waved away as Magical-Wagical Wooishness.
If you want to understand the properties of a crystal -- the real, physical properties of how it will behave when you zap it, polish it, wear it, build on it, break it, or keep it in your pocket -- you look at its structure.
Waaaayyyyyy easier subset.
You just have to pick the right axis.
Every crystal has translational symmetry: slide unit cell to repeat repeat repeat. Many ALSO have rotational or mirrored symmetry.
Like tessellations?
You'll love crystal structure.
A: Ahhh, but wait! Crystal structure influences shape, but what you see is the crystal habit. Crystal habit depends on:
crystal structure
if it's solitary or grows in packs
if it had room & resources to grow
Acicular crystals are fans of needles.
Globular crystals look like rock warts.
Cubic crystals look so fake it's awesome.
Dendritic crystals are fractals.
Colour (which is usually a useless lie)
Habit (shape)
Streak (colour of powder)
Cleavage or fracture (how it breaks)
Lustre (shiny!)
Density
Hardness
Random other shit like if it's lickable & tasty; if it's magnetic; if it glows when you bash it.
No magic necessary.
The science of caramelisation vs crystallisation of sugars is a huge part of baking desserts (and a challenge for how you could possibly make chocolate in space! qz.com/1680054/astron…).
It's still not magic.
Quartz, boring ol' quartz, is piezoelectirc: put it under mechanical stress and that fucker can generate electricity. WHAT?!
Calcite is birefringemt: it splits light. You can use that trick to find the direction of the sun on cloudy days.
Alexandrite takes "colour is a lie" to new lows by changing depending on the light source.
But I'll save it for @MineralCup in September.
Until then, browse mindat.org & minerals.net
This shrinks & grows as we find new wonkiness (space diamonds! quasicrystals! Pandoraite, which totally isn't doom-inspired!) or realize something's actually a variety of something else (ruby is fancy corundum!)
Physics, chemistry, and geology are scientific processes that work on other planets.
Based on composition, landscapes (geomorphology), processes we can see now, & any other context we can find, we can extrapolate the properties of minerals on other planets.
The season opener to Stargate: Universe has our heroes searching for carbonate rocks in desert-that-was-ocean to fix their spaceship air filter.
A: I feel soothed by smooth pebbles that have undergone enormous turmoil yet survived, inspired by how rocks quietly tell so many stories, & mesmerized by the geometric beauty of crystals.
Magic isn't necessary to like crystals.