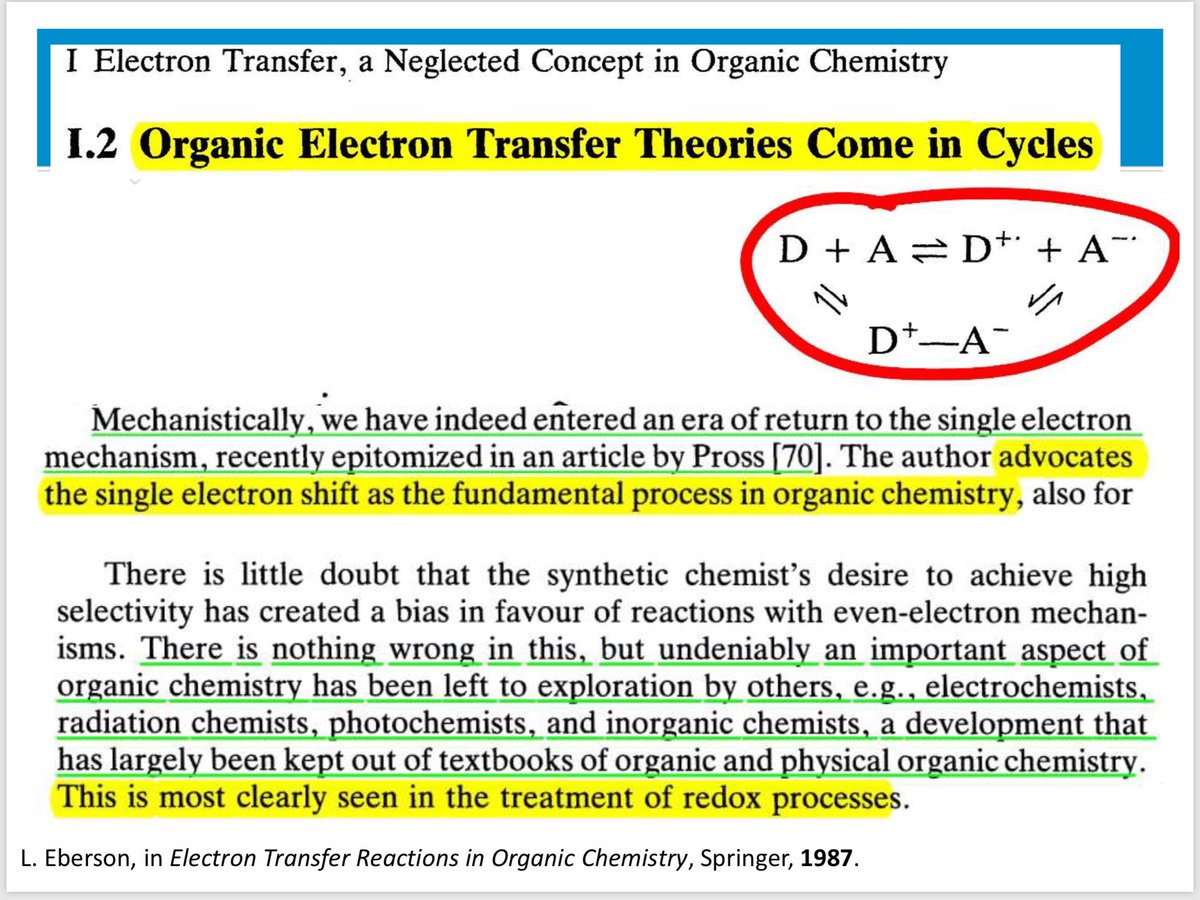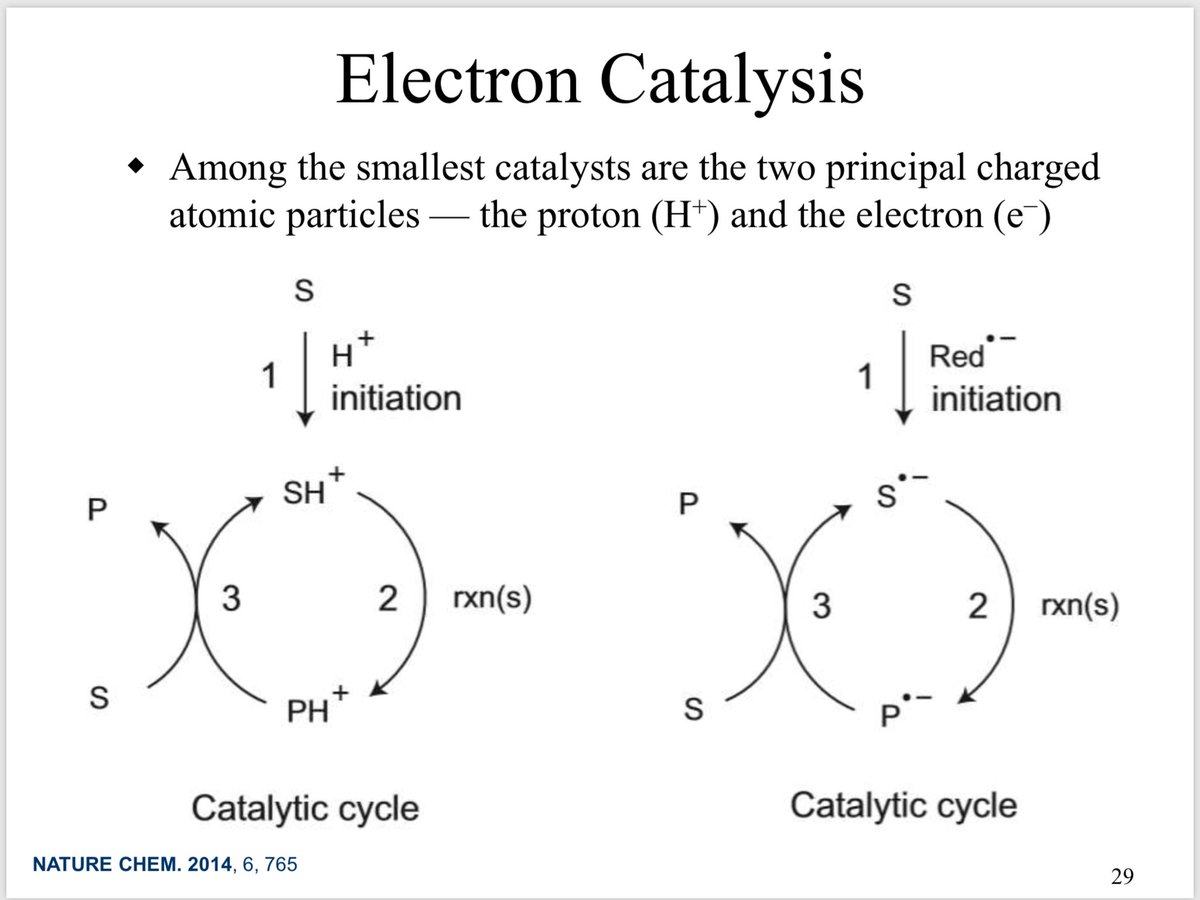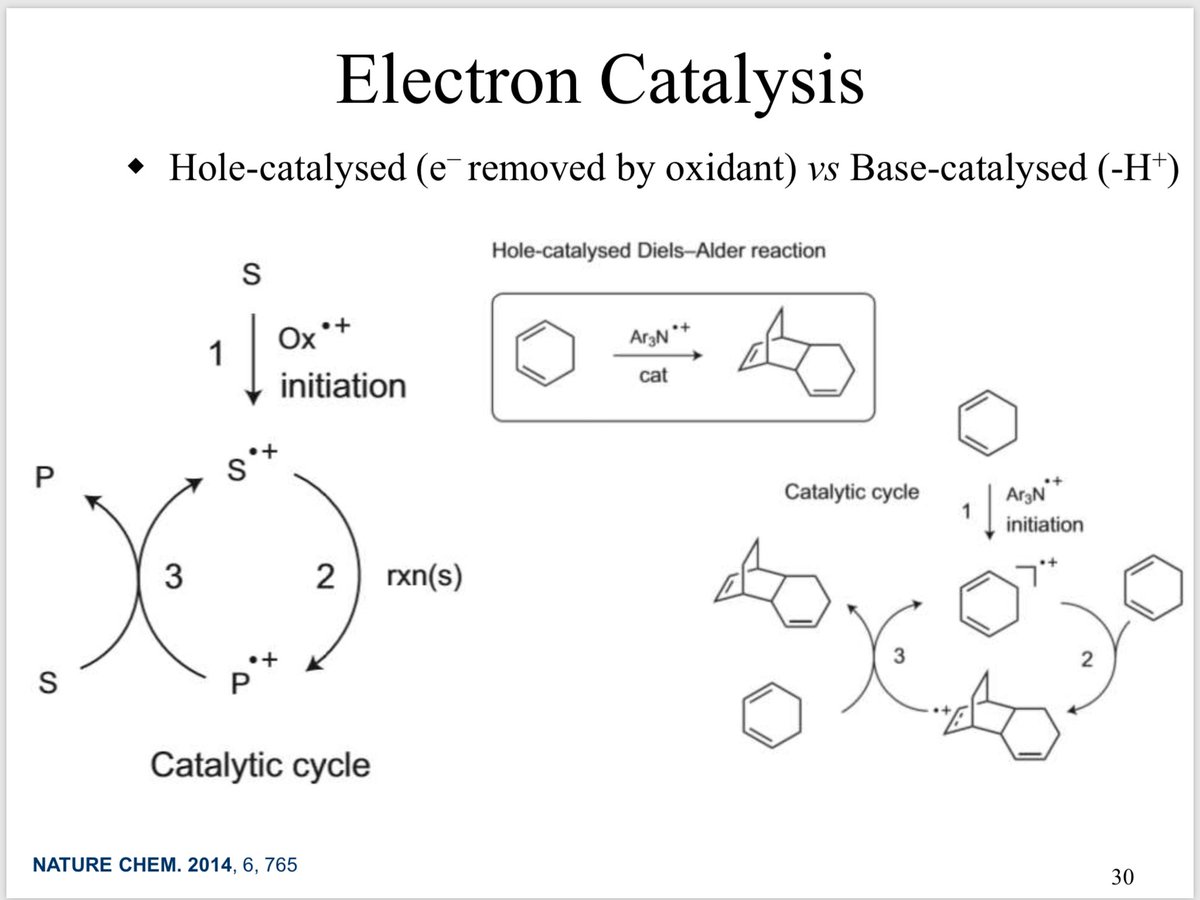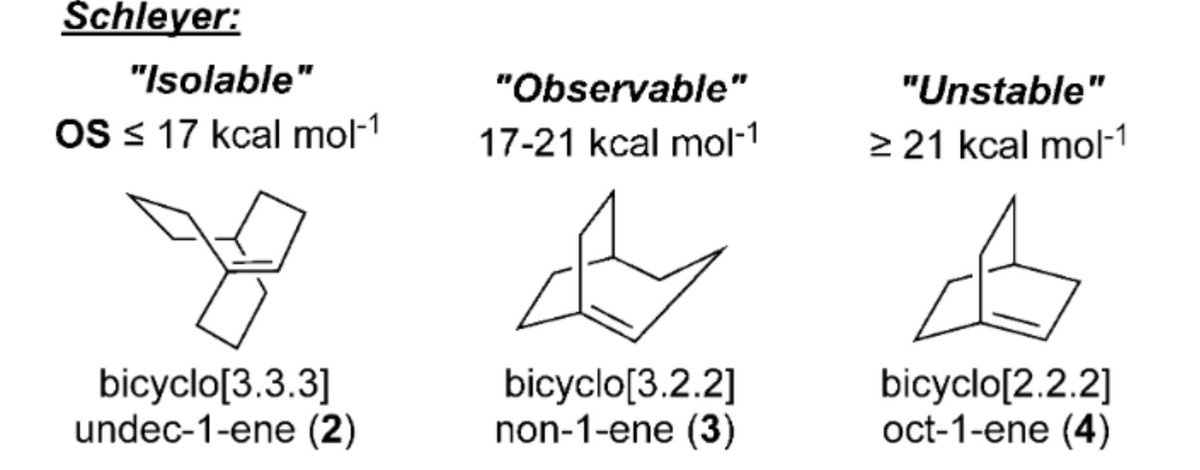
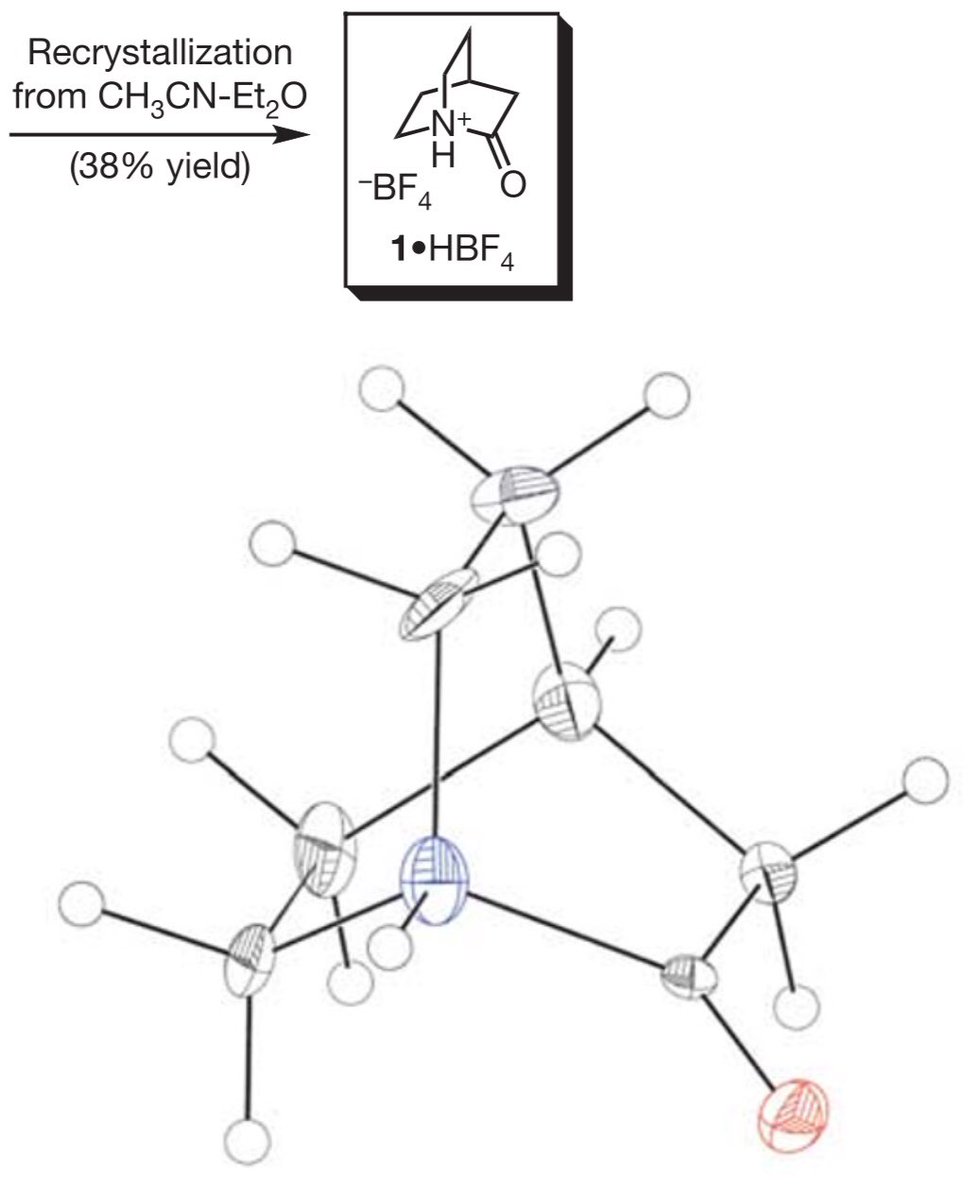
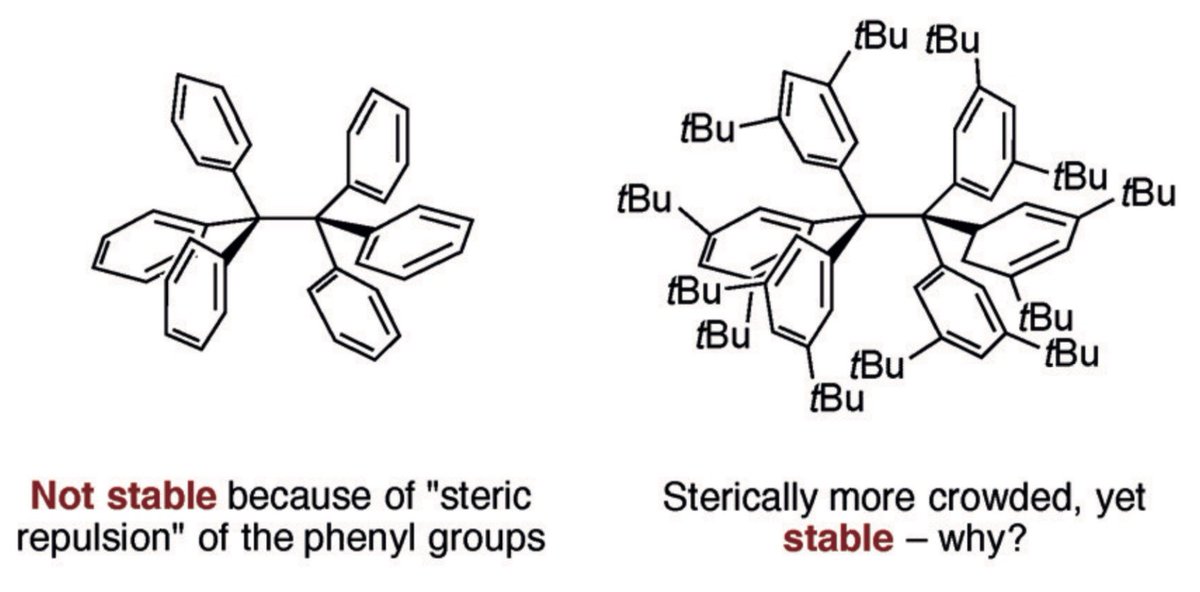
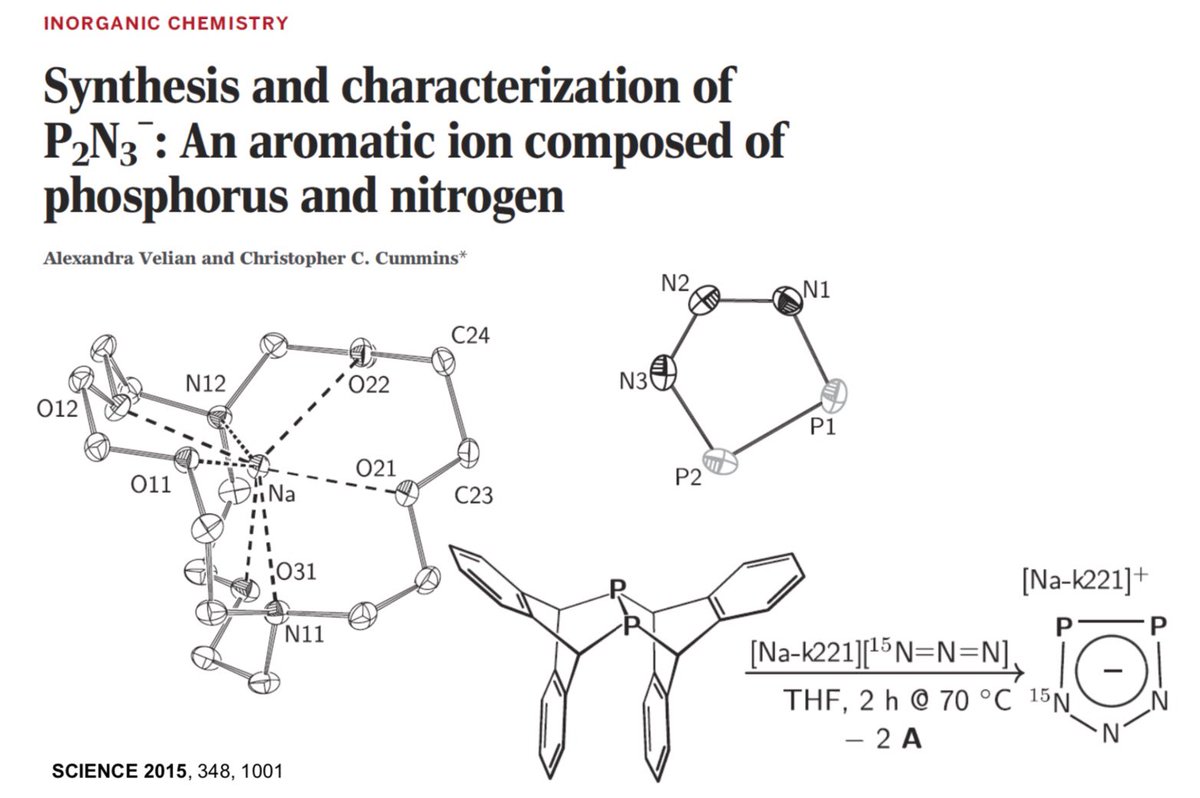
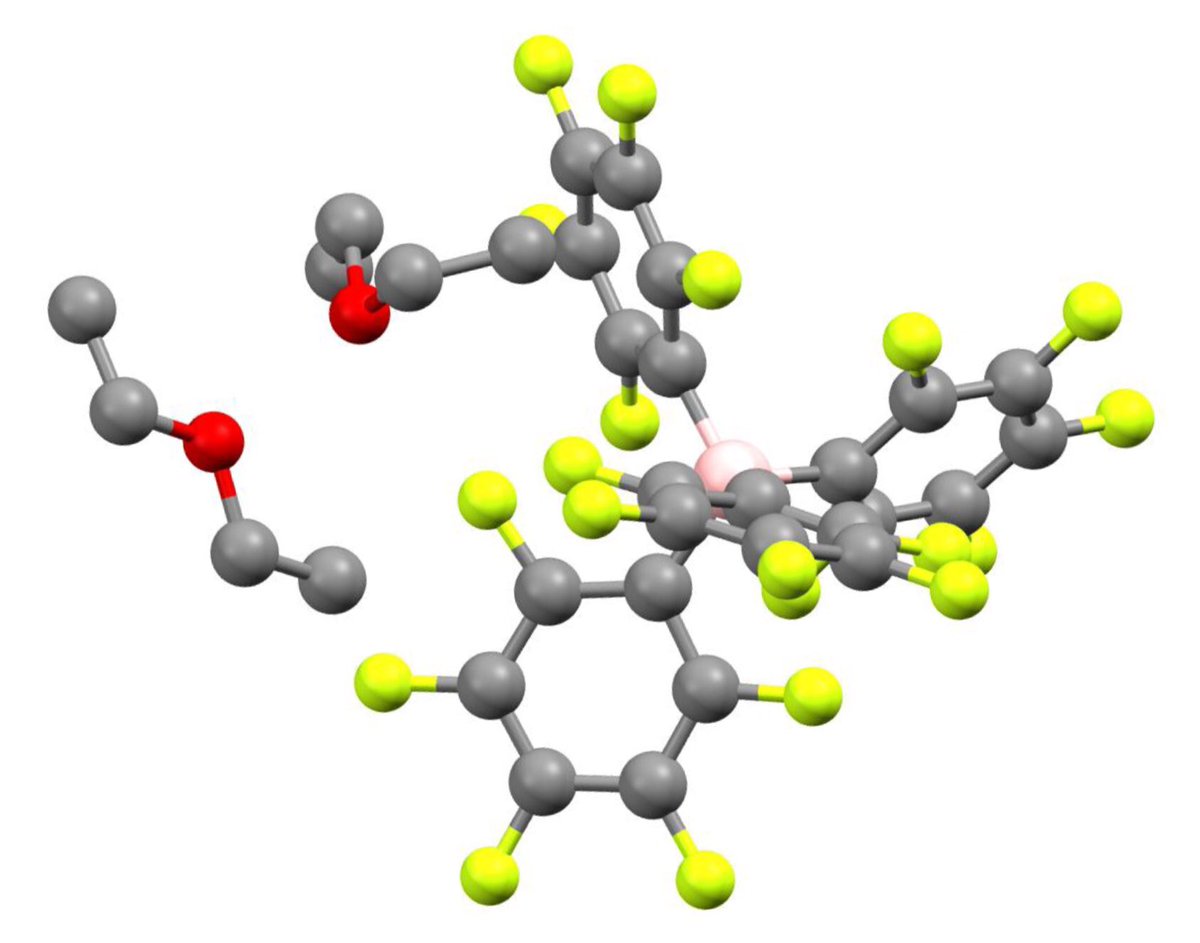
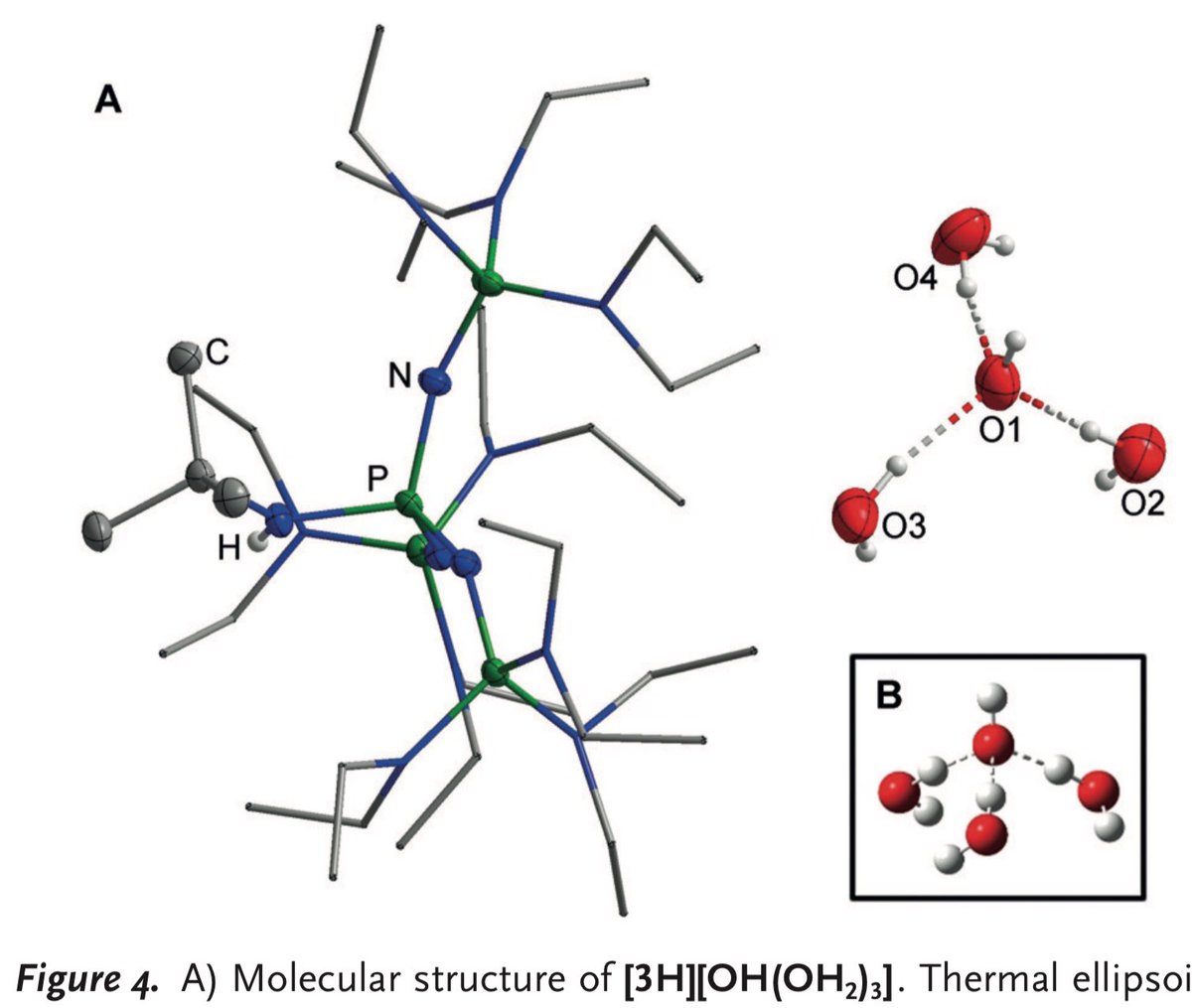
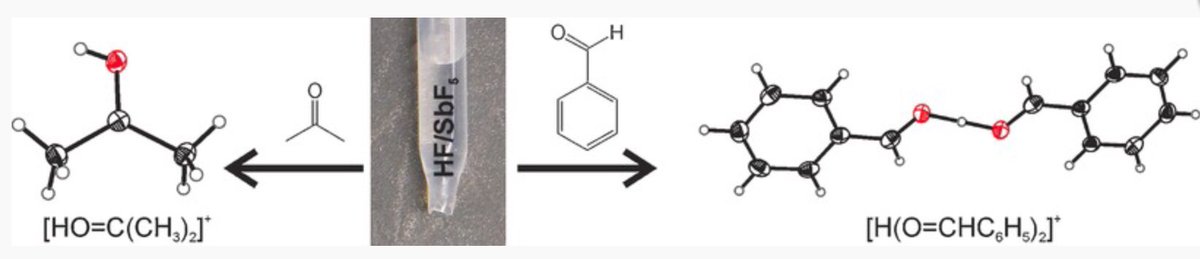
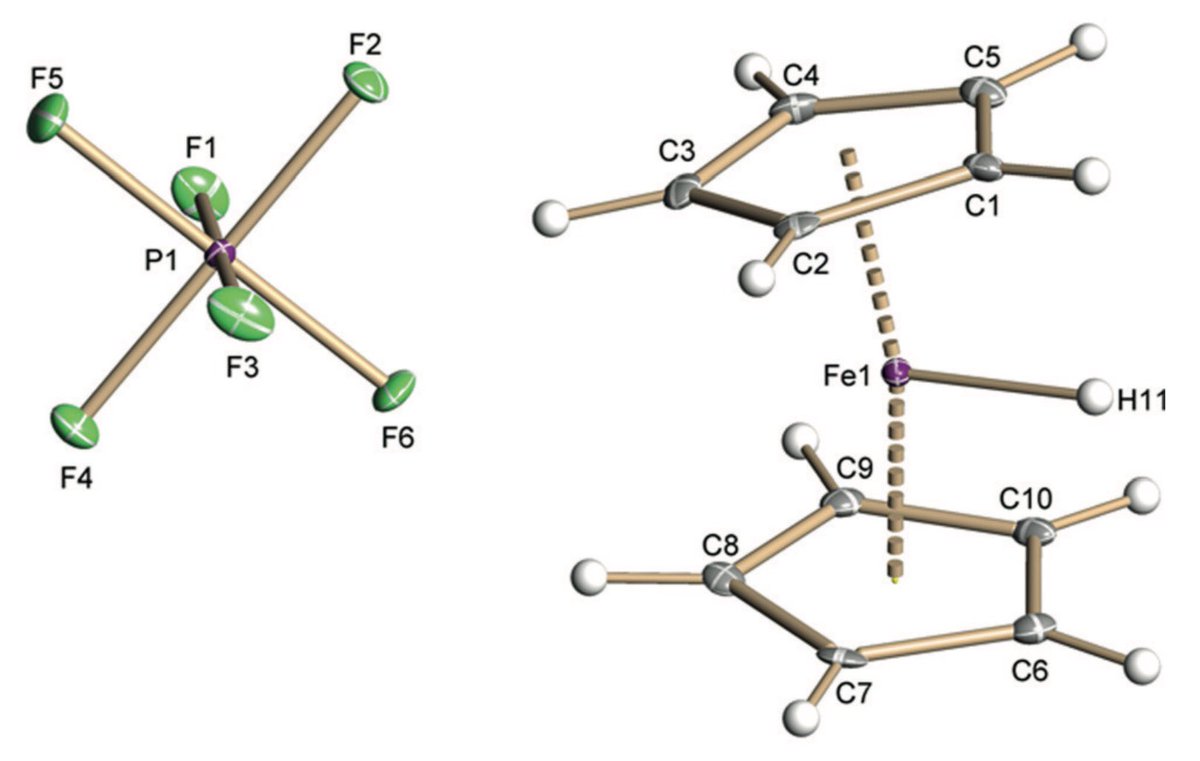
10.1039/C8SC03023E and protonated ferrocene by Meyer et al. doi.org/10.1002/anie.2…
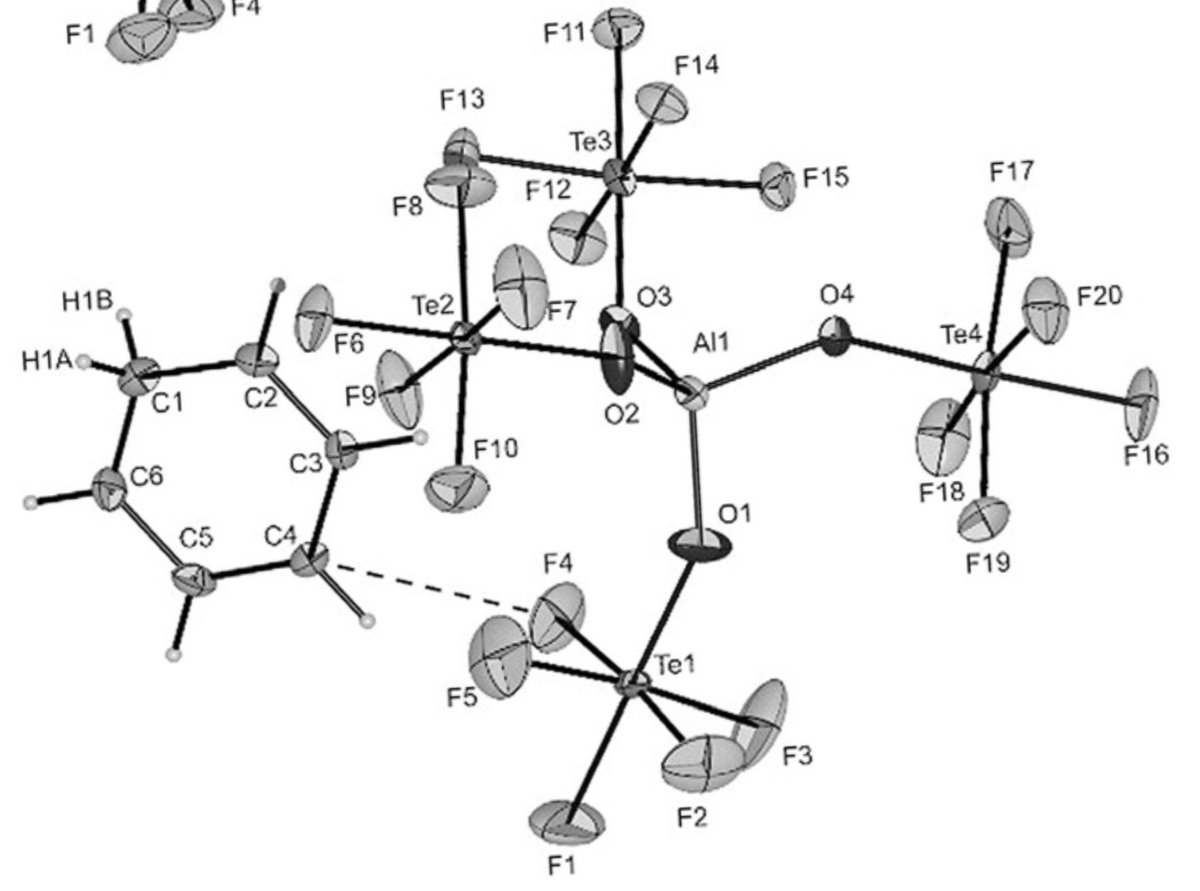
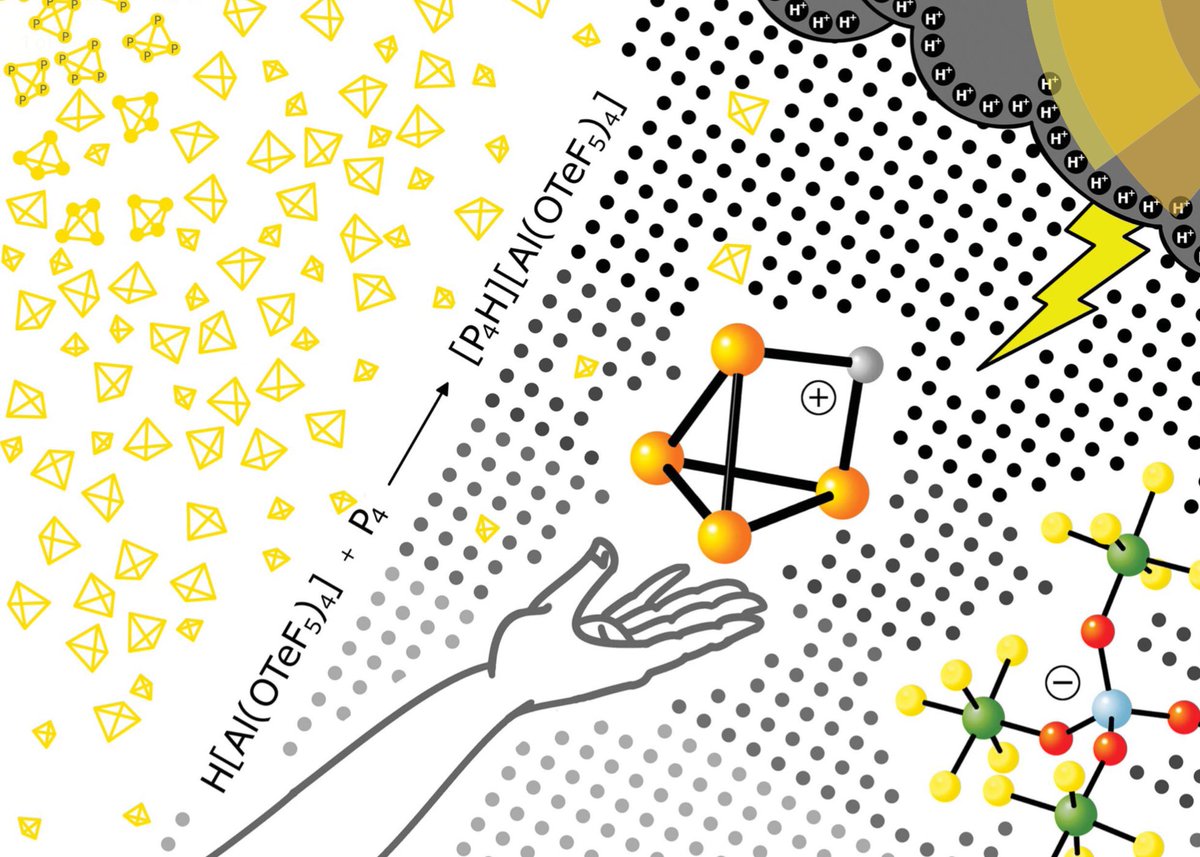
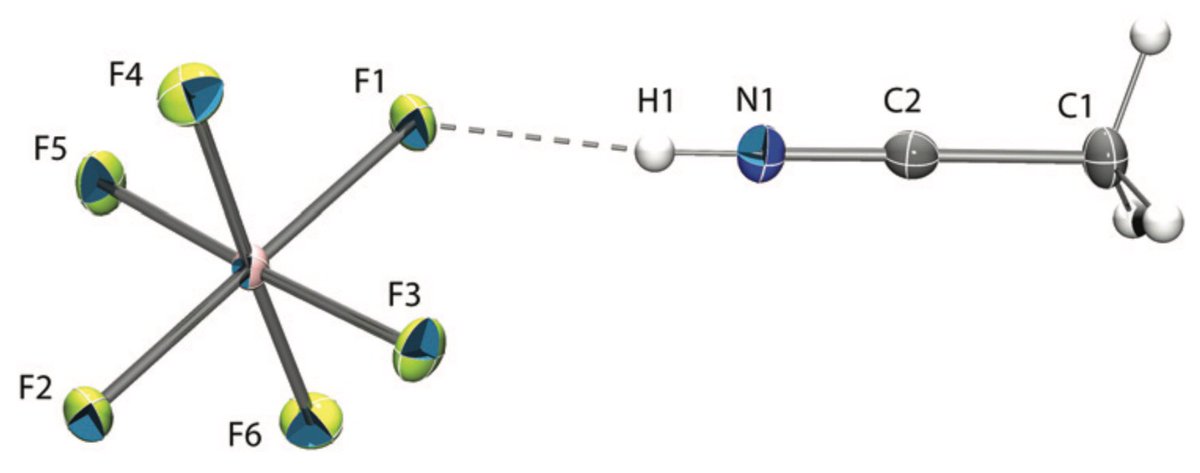
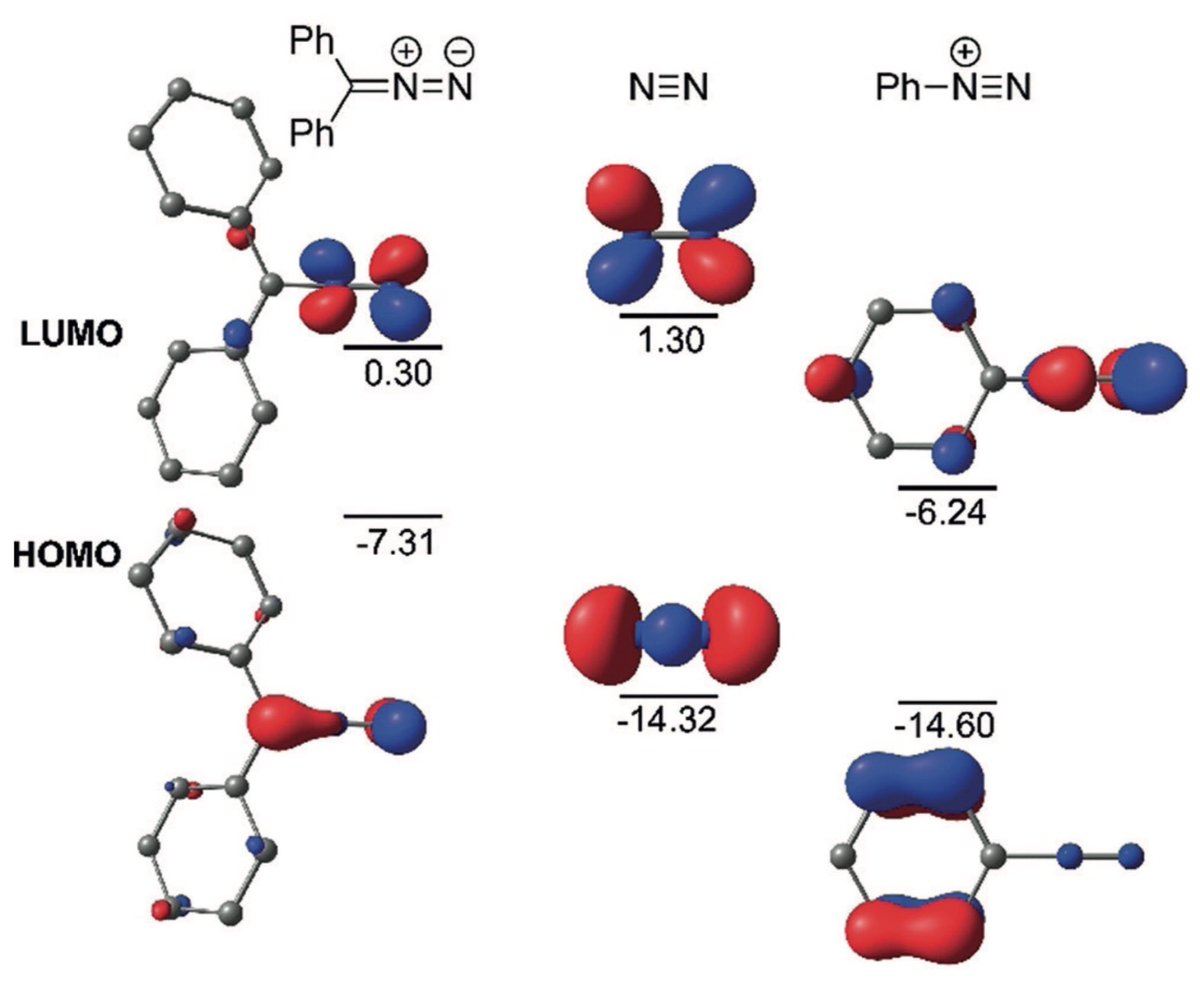

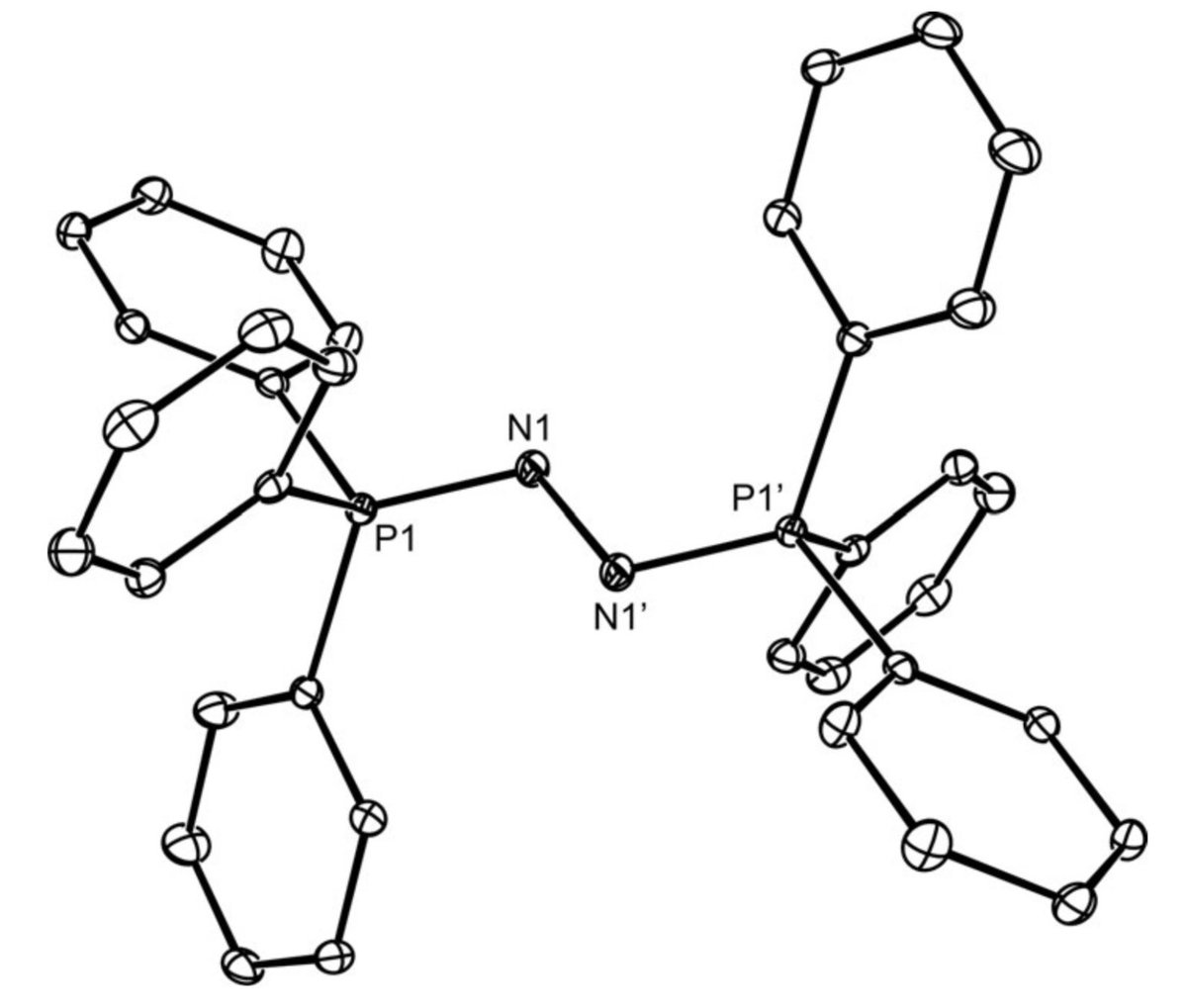
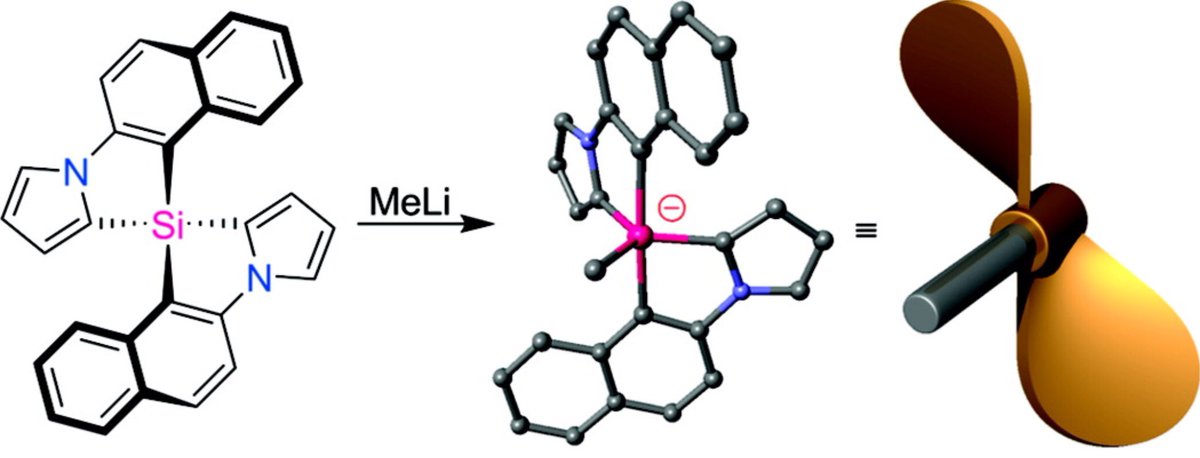
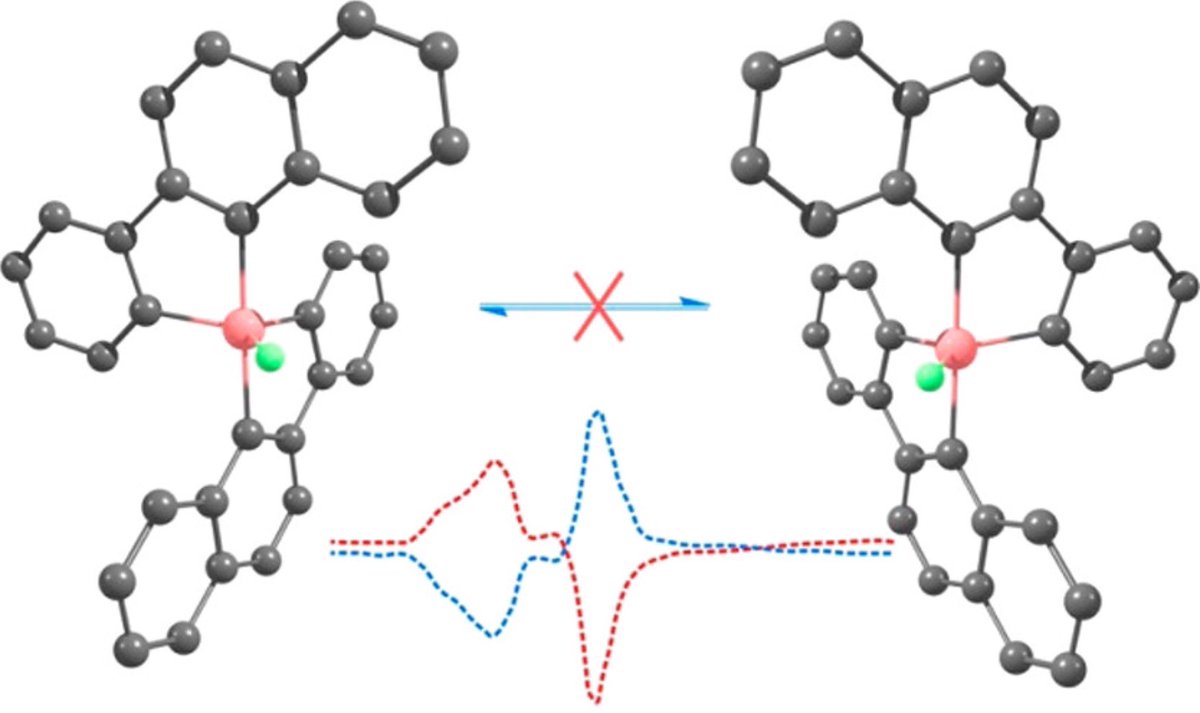
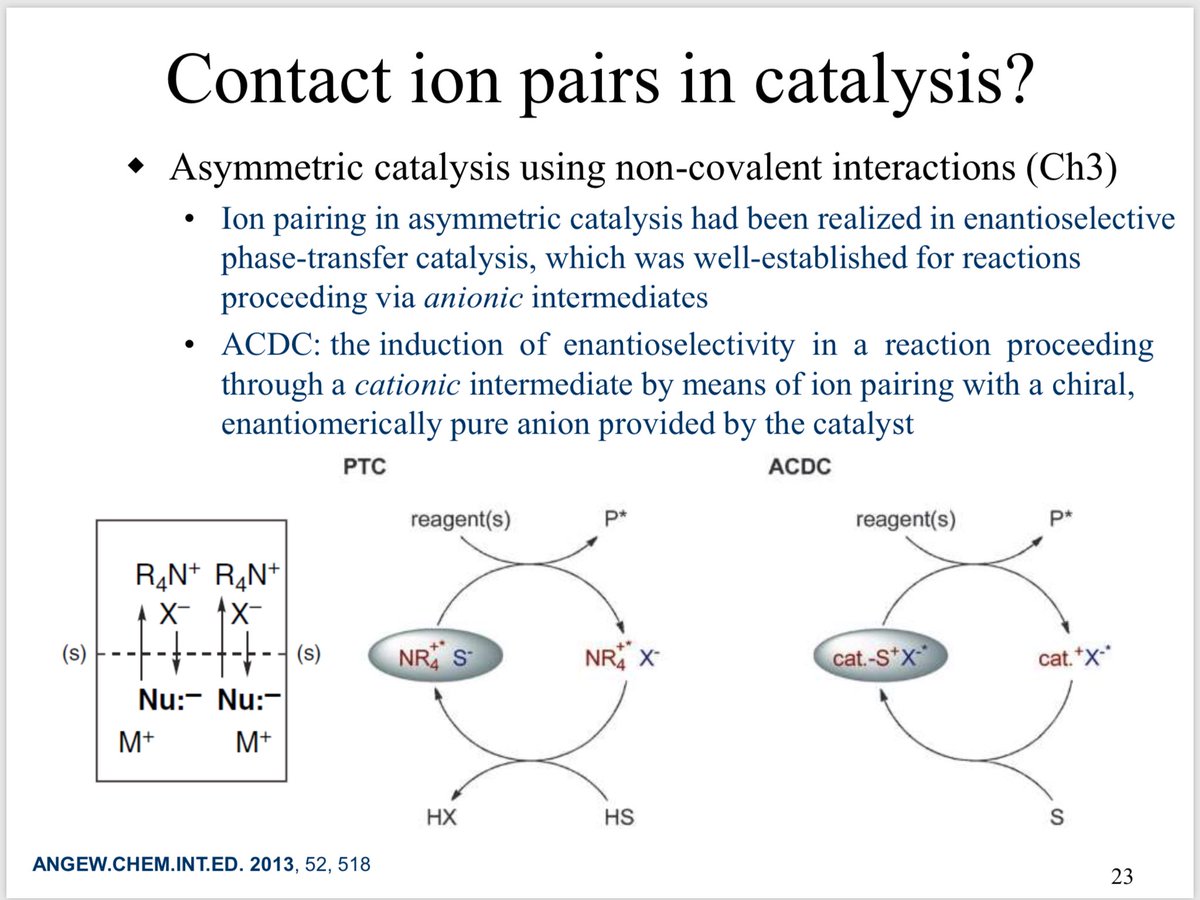

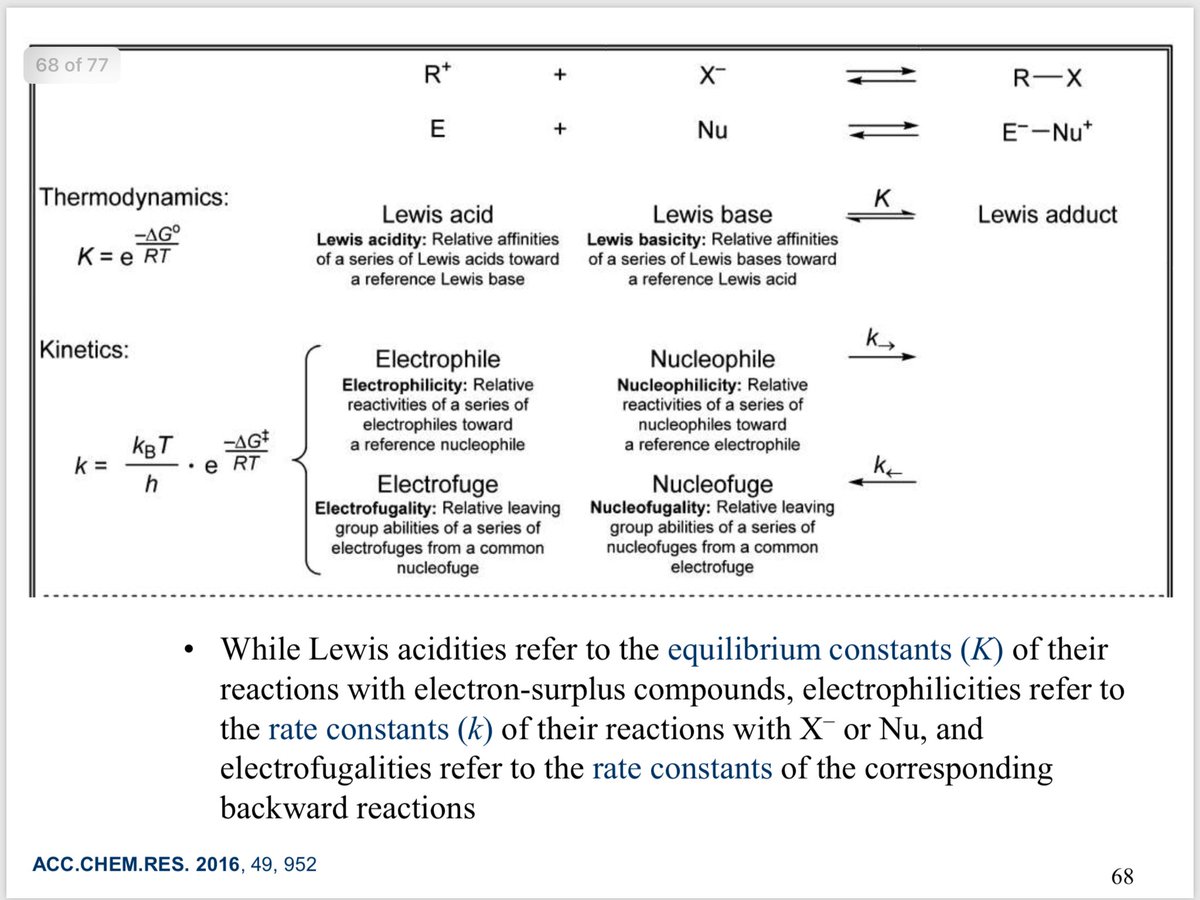
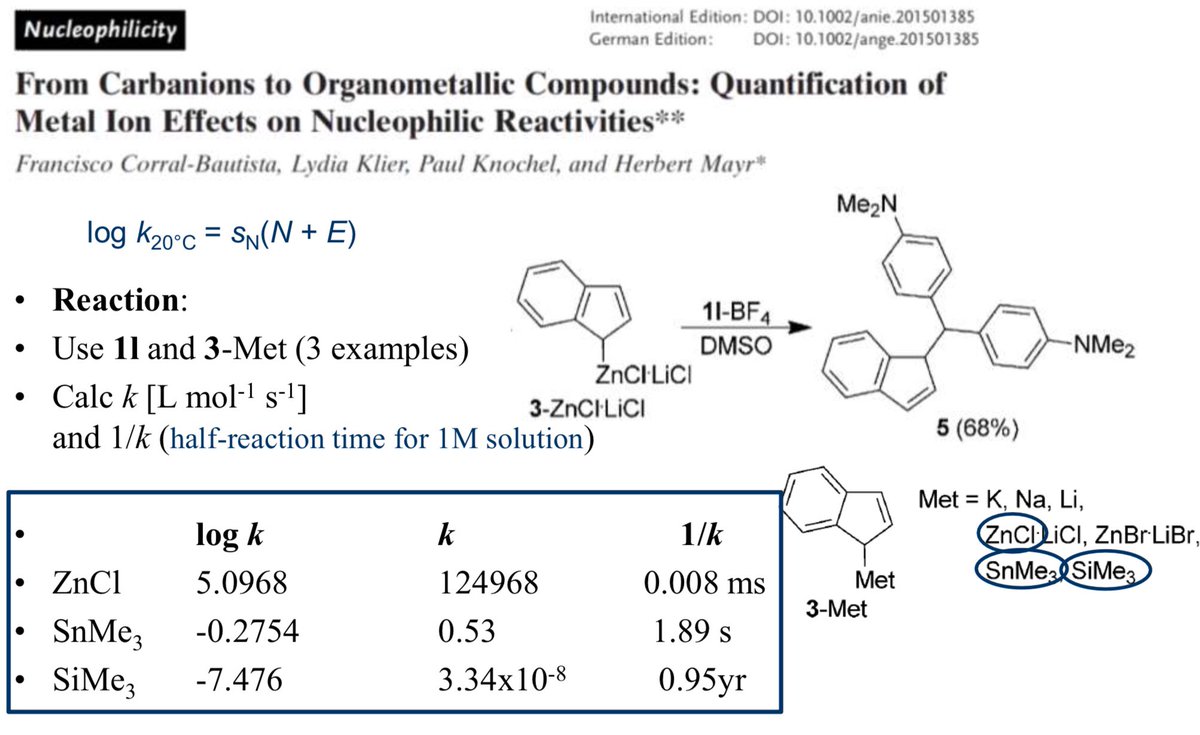
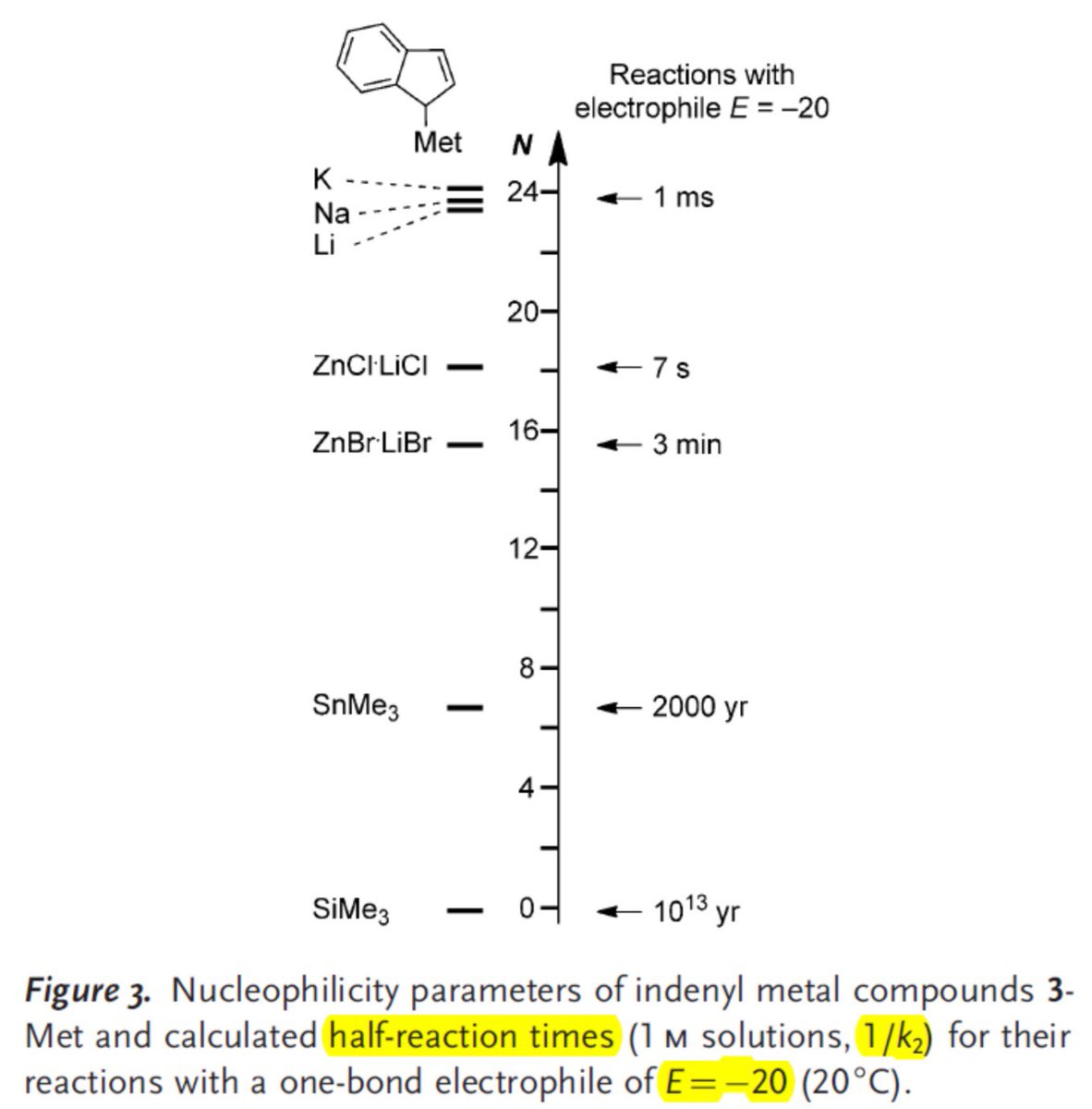

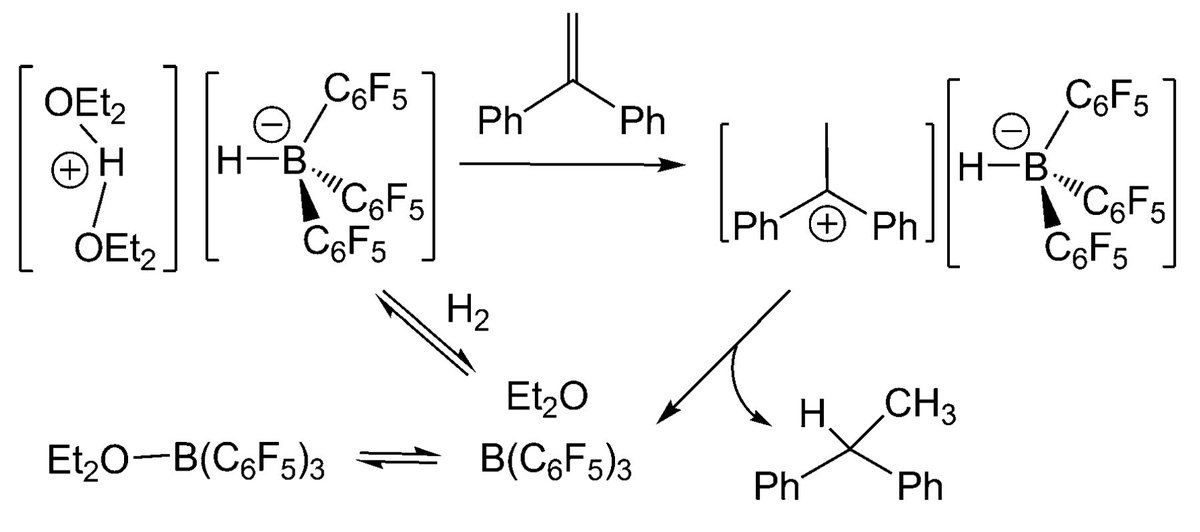
doi.org/10.1002/anie.2… @angew_chem. After heterolytic H-H cleavage using the weak Lewis base diethyl ether, the strong Brønsted acid [H(OEt2)2][H-BCF] is formed that can protonate 1,1‐diphenylethylene forming a stable carbocat.