The concept of enthalpy and entropy.
Dalam text book #Thermodynamics korang, ada je kan definition dia. Tapi i don't want to go through that.
ΔH tu enthalpy change. Normally korang akan jumpa benda ni dalam chemical reaction.
Tapi kalau korang tengok pada one particular compound, dia ada enthalpy, H dia sendiri. (again, bukan ΔH!)
Kalau korang pernah dengar term bond energy, ha, lebih kurang sama la tu konsep dia.
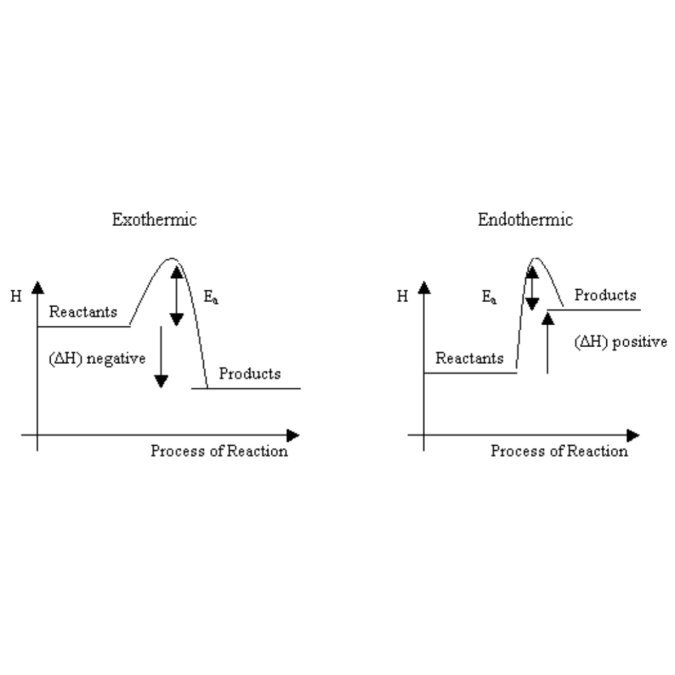
Systems lebih suka pada exothermic reaction. A reaction with -ve ΔH, reaction yang membebaskan haba.
Ye la, siapa je suka panas2 kan? Korang suka kalau haba dibuang, begitu juga at molecular level.
Kalau tengok gambar rajah kat bawah ni, going down the enthalpy level (being exothermic reaction) lagi mudah daripada nak gigih naik ke another enthalpy level (endothermic reaction)
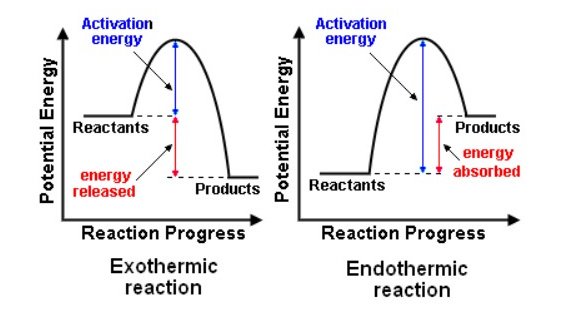
Tapi korang jangan confuse dengan activation energy pulak. Tu lain. Activation energy is the energy needed to start the reaction.
Just nak bagi "boost" untuk reaction jalan.
Actual statement of this law, korang boleh refer pada text book or google je.
In other words, Tuhan cipta dunia kita ni, dengan fixed amount of energy. Memang takkan tambah, takkan kurang. Tapi boleh ubah daripada satu bentuk ke bentuk yang lain.
Entropy ni, korang jangan confuse dengan energy pulak. Dia memang takde kaitan dengan energy, apatah lagi dengan enthalpy.
Entropy is a measure of randomness or disorder dalam satu system
Tu je. Ada saya mention pasal energy?
In other words, dia boleh tambah, boleh kurang. Entropy can be increased or decreased.
Systems lebih suka pada entropy yang lagi tinggi ie lagi berselerak, lagi random.
Ye la. Sama je la macam bilik korang kan. Lagi mudah untuk biarkan bilik berselerak rather than kena kemas everytime.
Selagi ada ruang, selagi tu system akan keep on bergerak and jadi berselerak, and increase in entropy sampai la dia dah takde kudrat dah untuk go further (achieve max entropy)
And this is our second law of #Thermodynamics
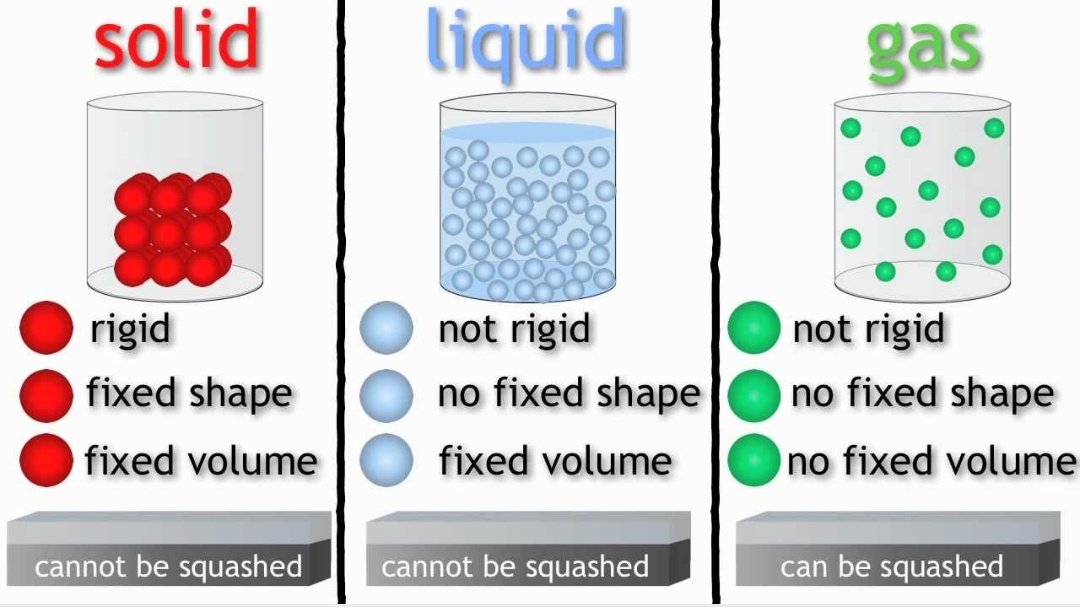
Sama jugak dengan entropy. Increase from solid, liquid and gas.
Gas has the highest entropy compare to solid and liquid (more random)
1. System suka pada minimum enthalpy
2. System suka pada maximum entropy.
Kalau korang dapat achieve 2 perkara ni, memang system akan suka gila, makanya reaction korang akan react spontaneously tanpa segan silu
Contohnya dalam synthesis of ammonia.
N2 (g) + 3H2 (g) ➡️ 2NH3 (g), ΔH = - ve
Dalam kes ni, enthalpy kau dah okay dah. - ve value. Tapi bila kau tengok pada entropy, entropy kau decrease.
So, tak best la. Ultimately, system akan go towards equilibrium antara minimum enthalpy and Max entropy.
The reverse of acid-metal reaction.
Mg(NO3)2 (aq) + H2 (g) ➡️ 2HNO3 (aq) + Mg (s), ΔH=+ve
Hence, this reaction is will not happen spontaneously or there will be no reaction.
Gibbs Free Energy.
ΔG = ΔH - T*ΔS
Kalau ΔG is negative, then the reaction is feasible and spontaneous and vice versa.
How to achieve this?
1. Negative ΔΗ, ie exothermic reaction
2. High temperature
3. Increase in entropy (reaction yang involve formation of more gas molecule or aqueous solution)
Next, what happened if your ΔG = 0?
What does it means?
Recap balik pasal ammonia synthesis tu
Memang tak leh nak go dah.