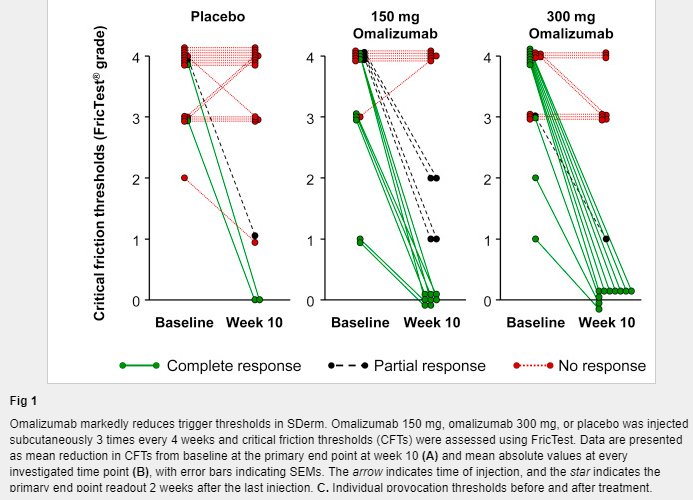
Does that matter commercially? No. Takzhyro is 85-90%. (1/2)
Cash as of 3/31 at $128m, burning $30m/q, and $80m debt, so < $1/sh
The issue with HAE is that attacks can be fatal, especially if laryngeal. Need really good coverage to feel “safe”. Tough to prescribe when comp has 85%+ efficacy
Monumental education effort required,which translates to a lot of capital needed (assuming this even gets approved)
- Was the ph2 truly unblinded?
- Doesn't it have a distinct strong unpleasant taste? Is it true patients can't mix it or drink anything 30 min before or after?