Why is cancer associated with elevated lactate dehydrogenase (LDH)?
The answer is cool and provides insights into cancer that may not be obvious at first. So, what do you think?
What do you think provides the best explanation?
Before getting to the answer, let's establish that cancer is associated with increased serum LDH levels.
This link has been observed for decades. Here is one of the earlier studies, from 1954.
ncbi.nlm.nih.gov/pubmed/13190533
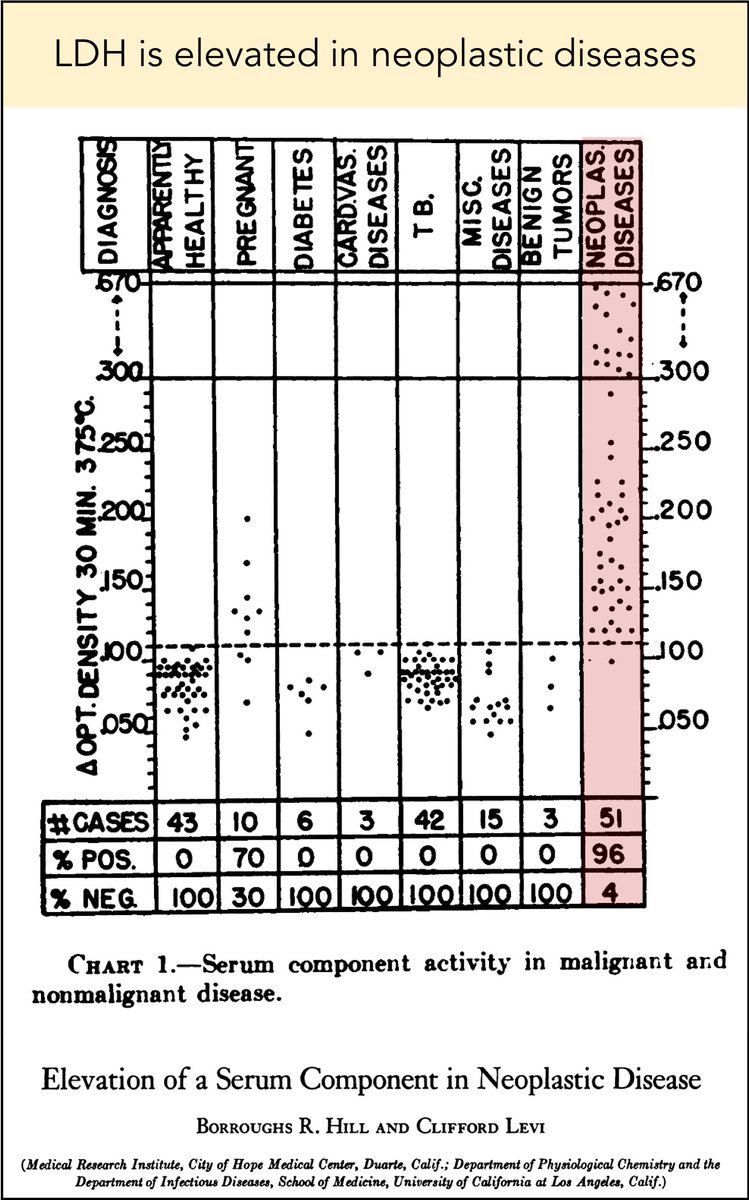
There are a number of potential explanations for this observation.
I often hear that LDH is increased in cancer due to "increased turnover". But, cancer is associated with increased proliferation and decreased apoptosis.
Something else is going no.
ncbi.nlm.nih.gov/pubmed/21943236
An alternative explanation: cancer overexpresses (i.e., makes more) LDH.
This overexpression has been demonstrated, with control by both c-myc and hypoxia-inducible factor 1 (HIF-1).
ncbi.nlm.nih.gov/pubmed/9192621
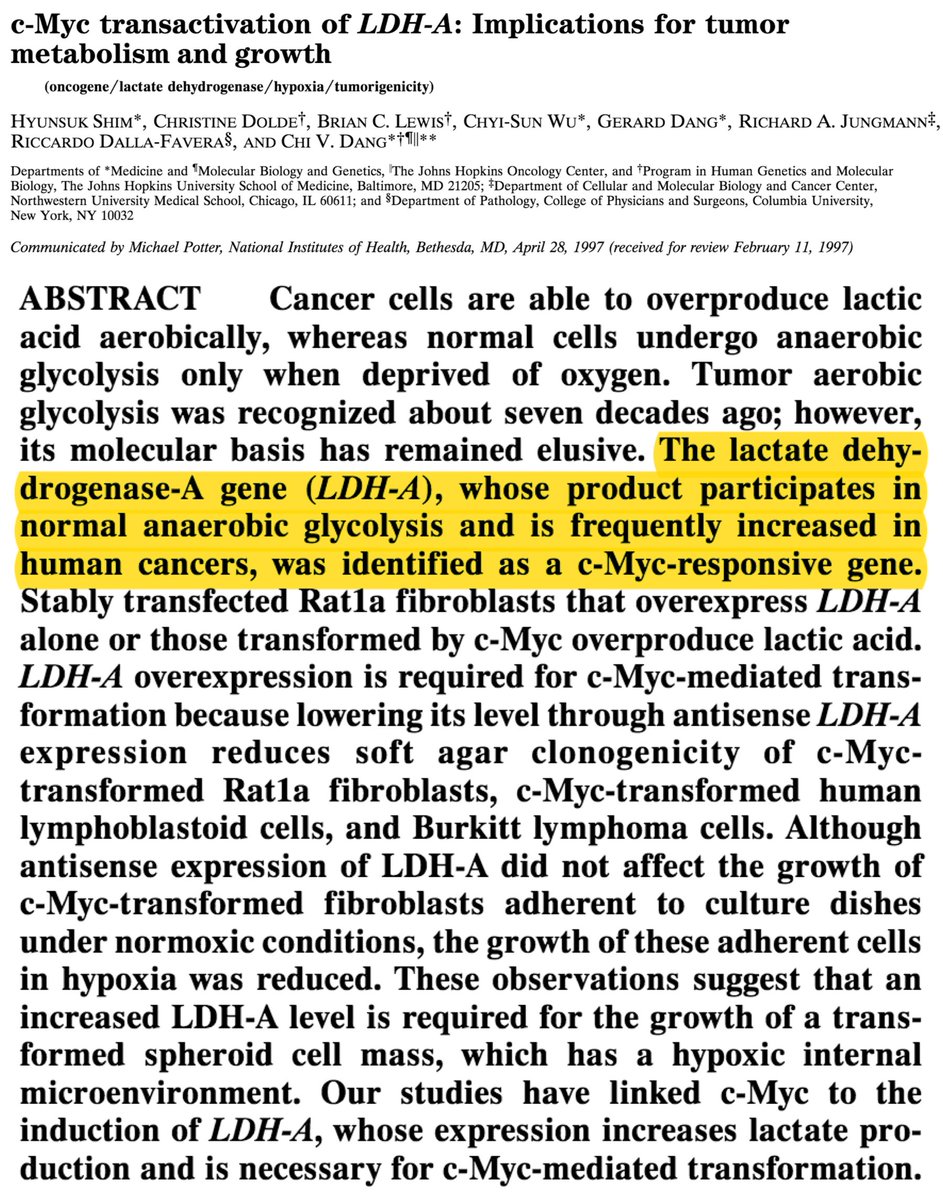
In order to understand the clinical implications of LDH overexpression, recall it is an enzyme that:
reduces pyruvate to lactate...
...under conditions of high glycolytic flux...
ncbi.nlm.nih.gov/pubmed/27993896
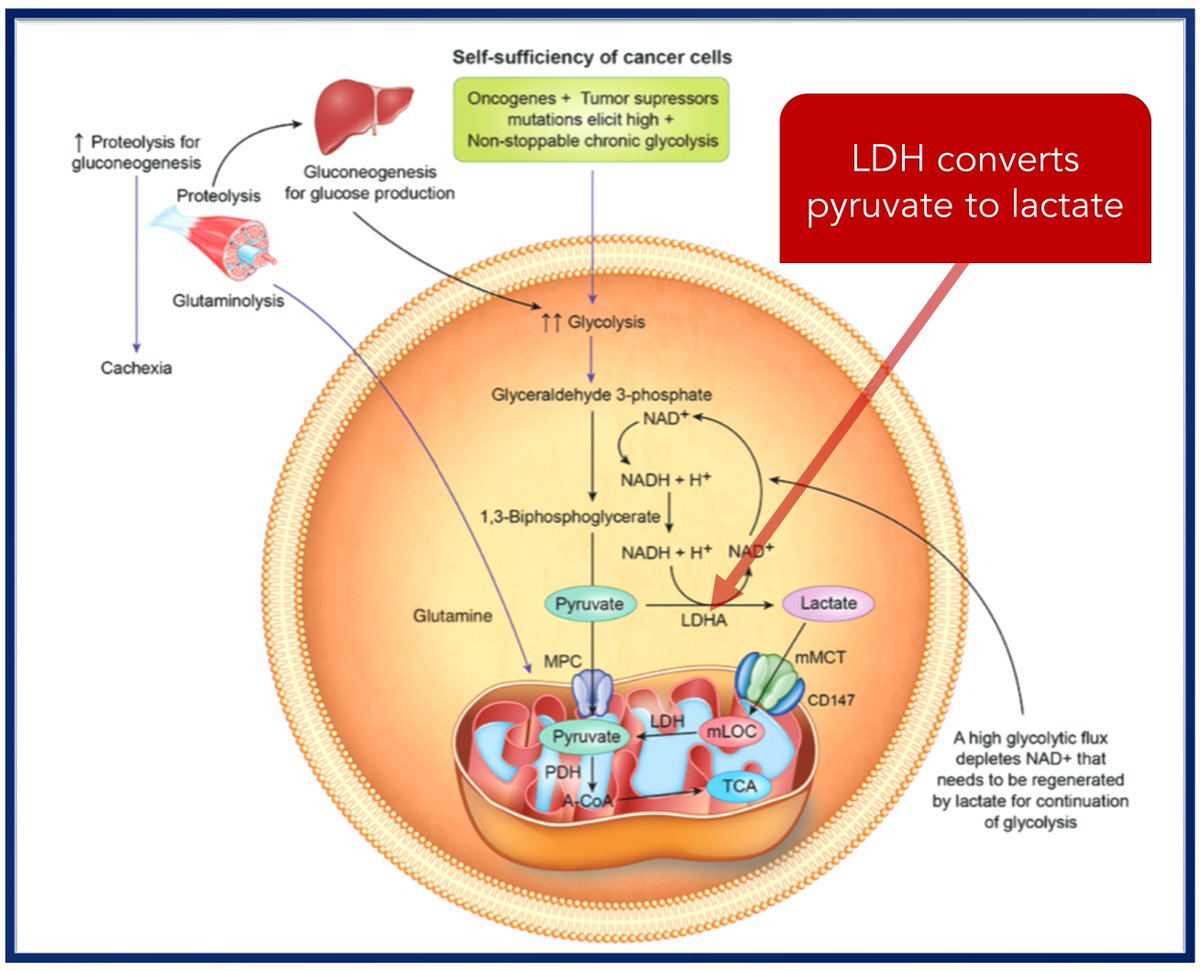
...conditions like cancer!
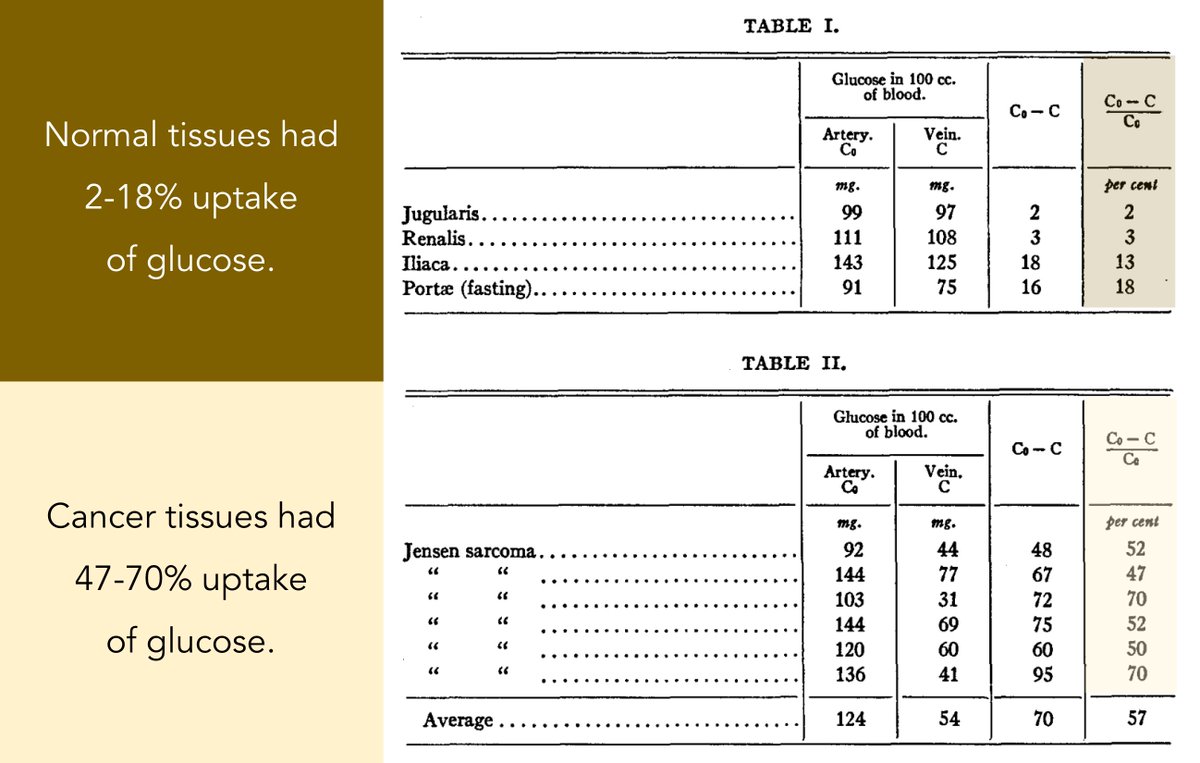
Nearly 100 years ago Warburg and Minami found that cancer cells were characterized by accelerated glycolysis and excessive lactate formation even under fully oxygenated conditions.
This has been called the Warburg Effect.
link.springer.com/article/10.100…

Warburg also demonstrated that glucose update is 47-70% in tumor cells versus 2-18% in non-tumor cells.
And: tumor cells convert 66% of glucose to lactate.
ncbi.nlm.nih.gov/pubmed/19872213
So, cancer cells overexpress LDH, overproduce lactate, and overconsume glucose.
Three questions follow from these observations:
➢ How do cancer cells do this?
➢ Why do they do this?
➢ What are the clinical implications?
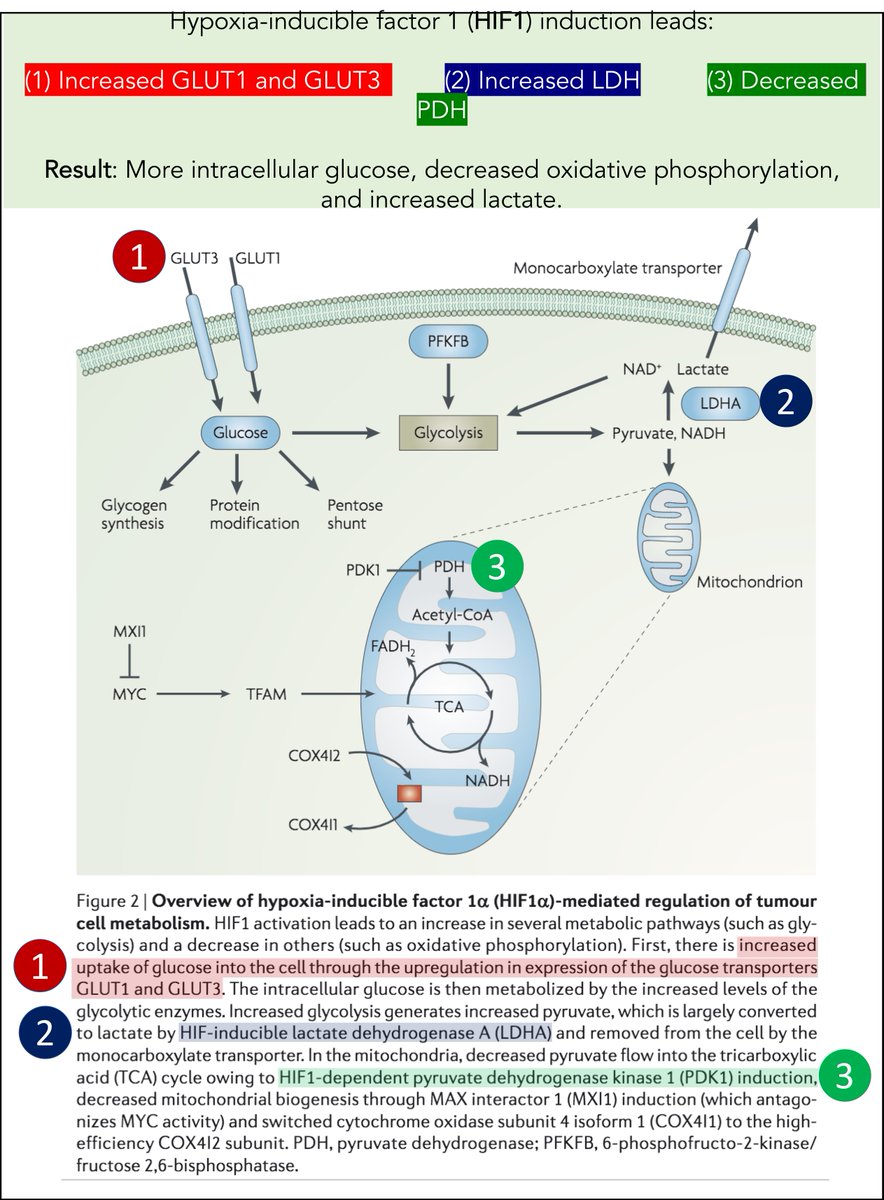
First, how.
As noted above, overexpression of c-Myc and HIF-1 is seen in cancer. This leads to a shift to glycolysis.
For example, HIF-1:
↑GLUT-1 (↑intracellular glucose!)
↓pyruvate dehydrogenase (↓oxidative phosphorylation!)
↑LDH (↑lactate)
ncbi.nlm.nih.gov/pubmed/19143055
Clinical implication #1:
The reason that cancer lights up on a PET-CT is that cancer cells take up more glucose than the surrounding tissues.
And they take up more glucose for the reasons noted in the preceding tweets.
ncbi.nlm.nih.gov/pubmed/24947987
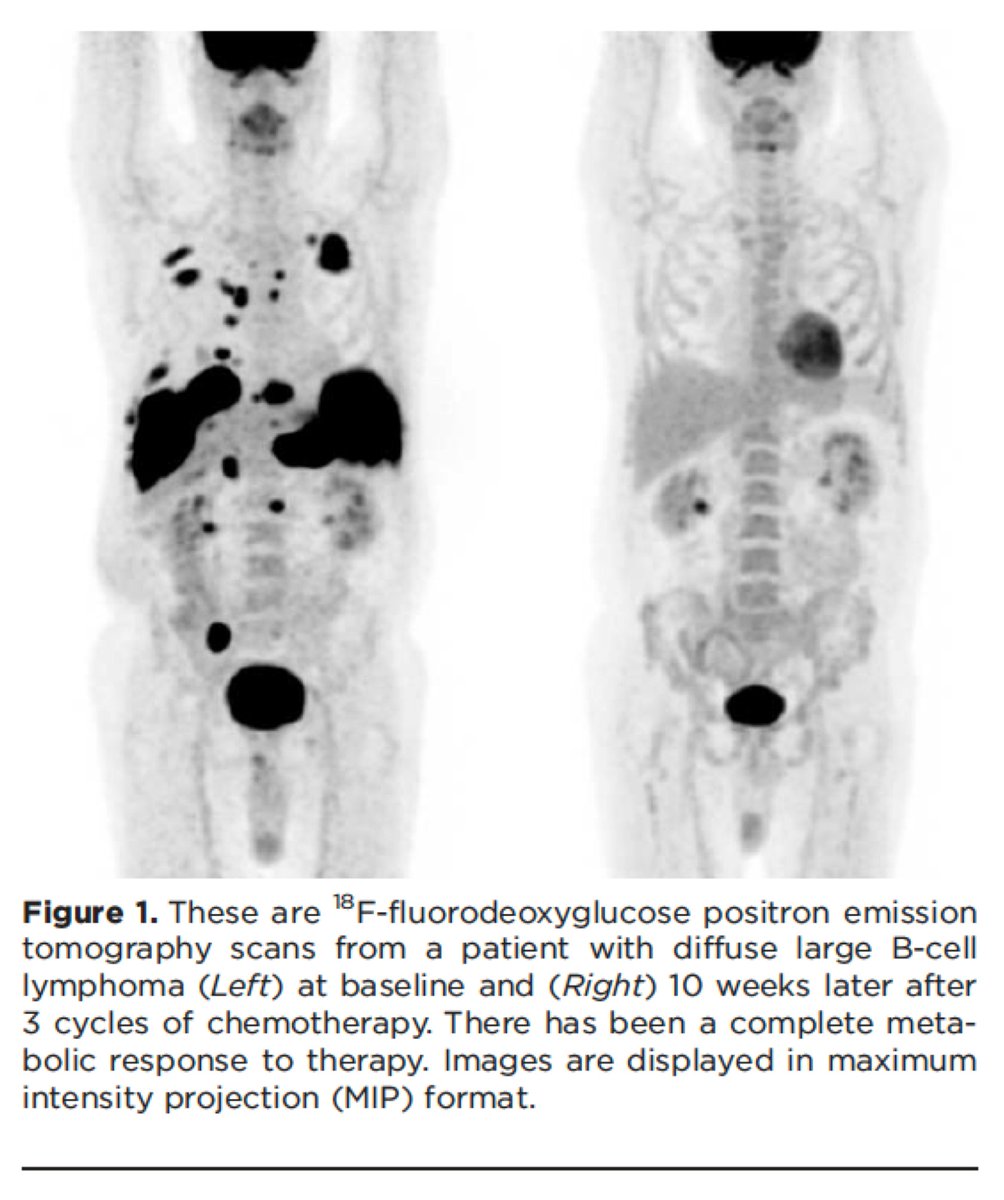
Clinical implication #2:
Cancer cells use glucose and produce lactate, which is sent to the liver and recycled back to glucose. This is energy-dependent. To keep up, fat and muscle are catabolized.
Result: one potential cause of cancer cachexia.
ncbi.nlm.nih.gov/pubmed/29609556
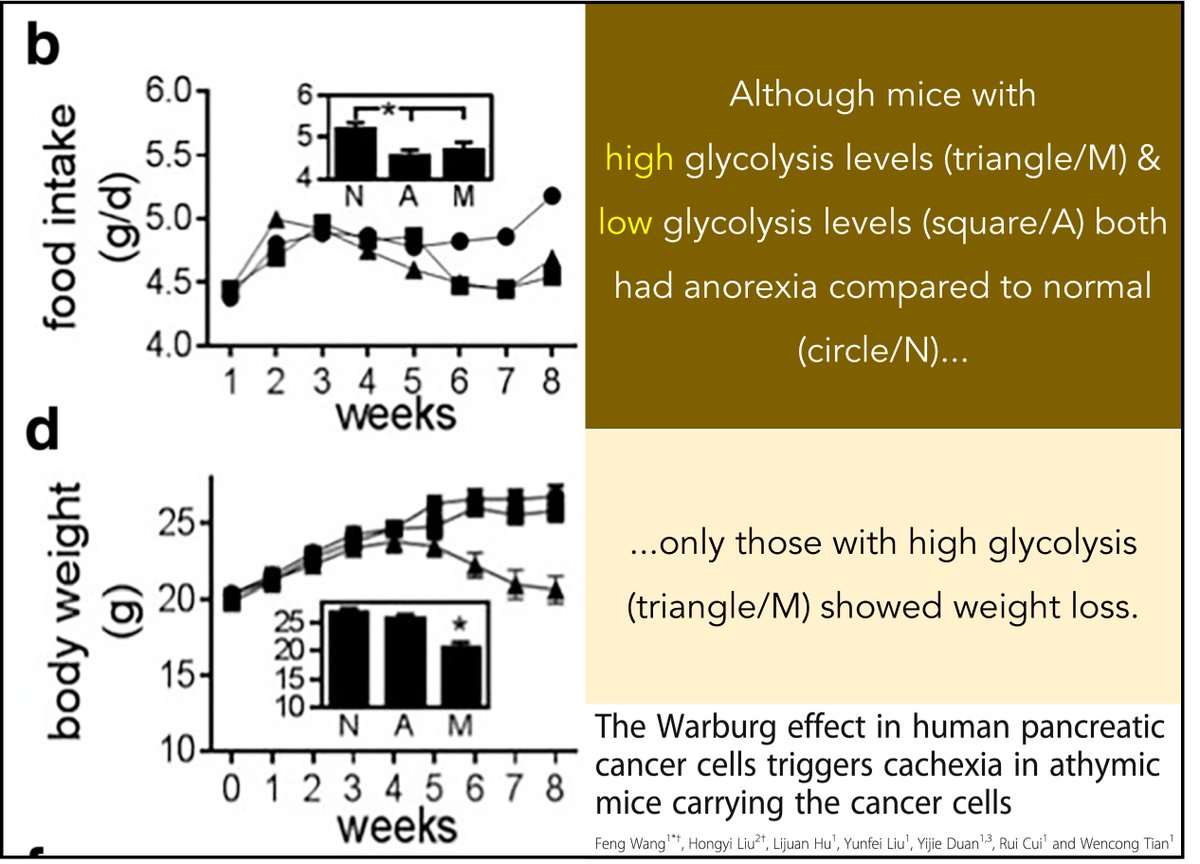
But WHY do cancer cells prefer glycolysis to oxidative phosphorylation (given that the latter produces so much more ATP per glucose)?
Cancer cells prefer glycolysis for many reasons.
A lot is driven by a desire for lactate.
Remember, ↑LDH leads to ↑lactate. Much of this lactate is shuttled OUT of the cancer cell, leading to an acidic local environment.
This may benefit tumors.
ncbi.nlm.nih.gov/pubmed/24367336
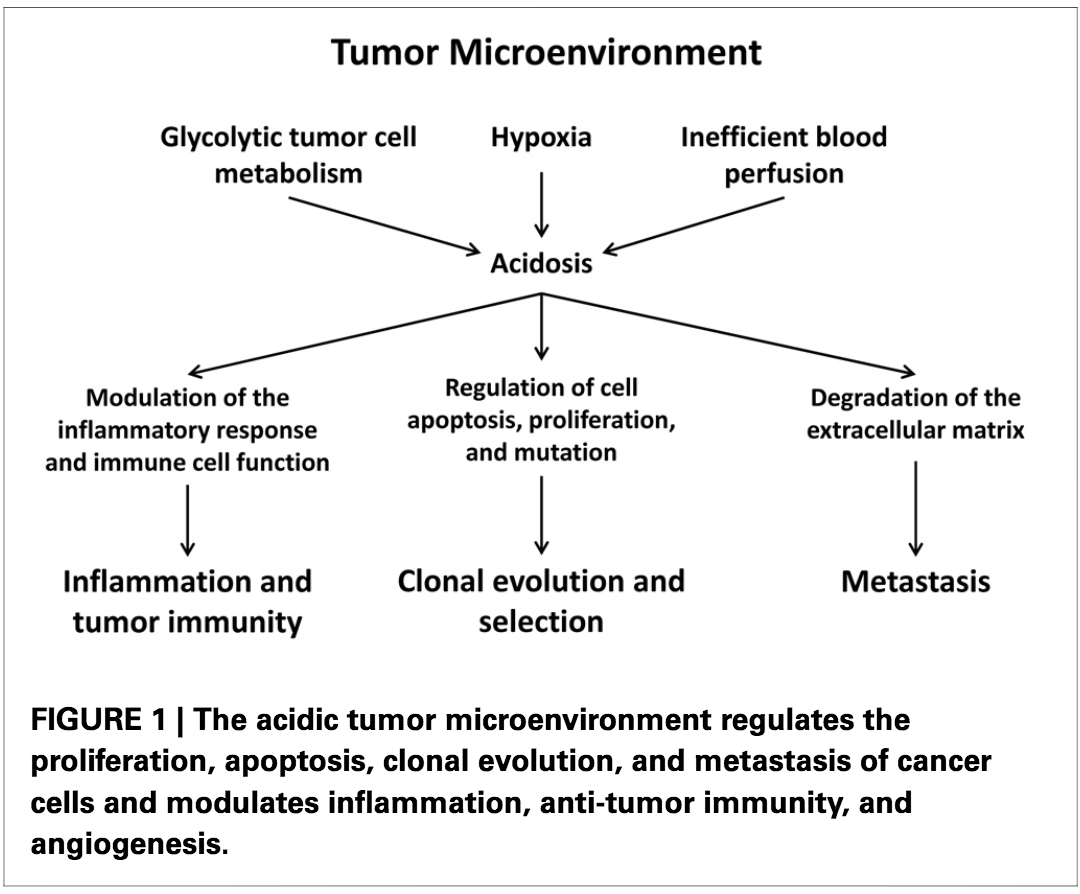
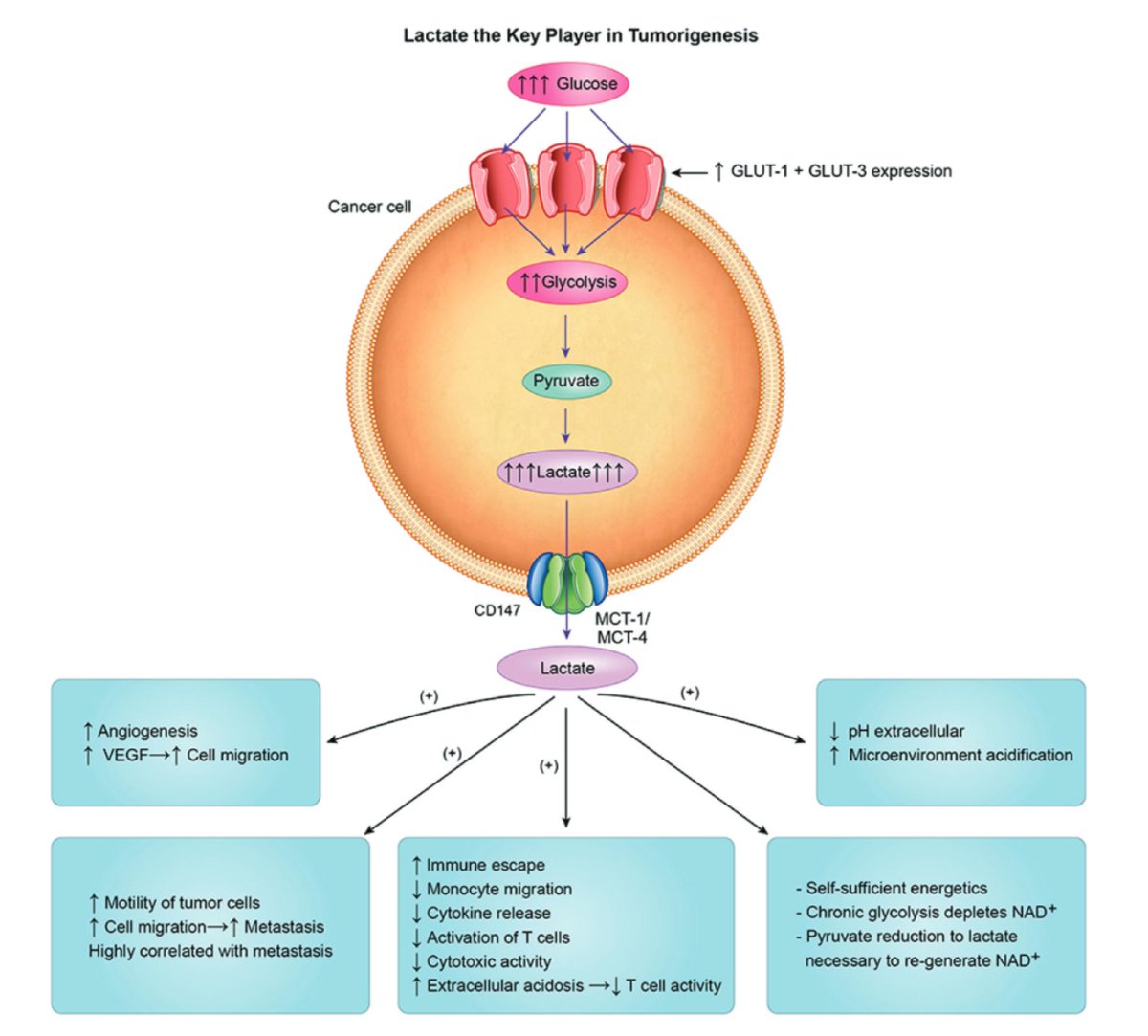
There are other potential benefits of all the extra lactate:
↑angiogenesis
↑tumor migration
↑immune escape
It seems that lactate is not just a byproduct of the Warburg effect, but potentially its intent.
ncbi.nlm.nih.gov/pubmed/27993896
Before closing, let's ask the original question once more.
💥Why is cancer associated with elevated lactate dehydrogenase (LDH)?💥
➢ Cancer cells preferentially use glycolysis
➢ The shift to glycolysis is mediated by c-myc, HIF-1 (and others)
➢ LDH is overexpressed in cancer, leading to more lactate production (and high serum levels)
➢ PET-CT makes use of this