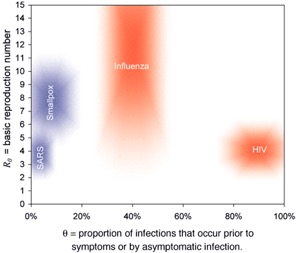
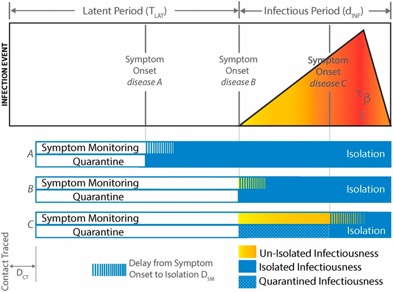
a) unreliable reporting (missing cases) -can lead to biased estimates of R0 (up or down) if there are changes in proportion of cases detected or in delays in reporting
b) uncertain serial interval (time step over which to calculate the rate)
nextstrain.org