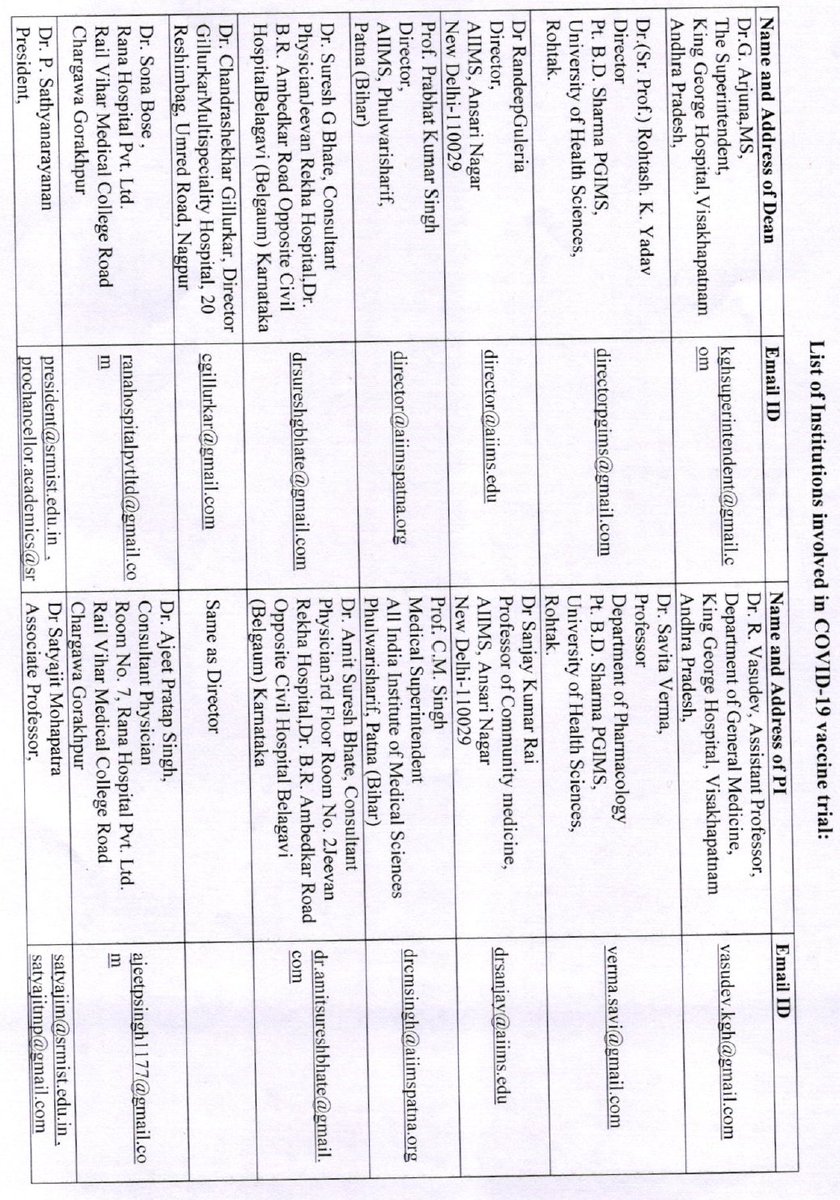
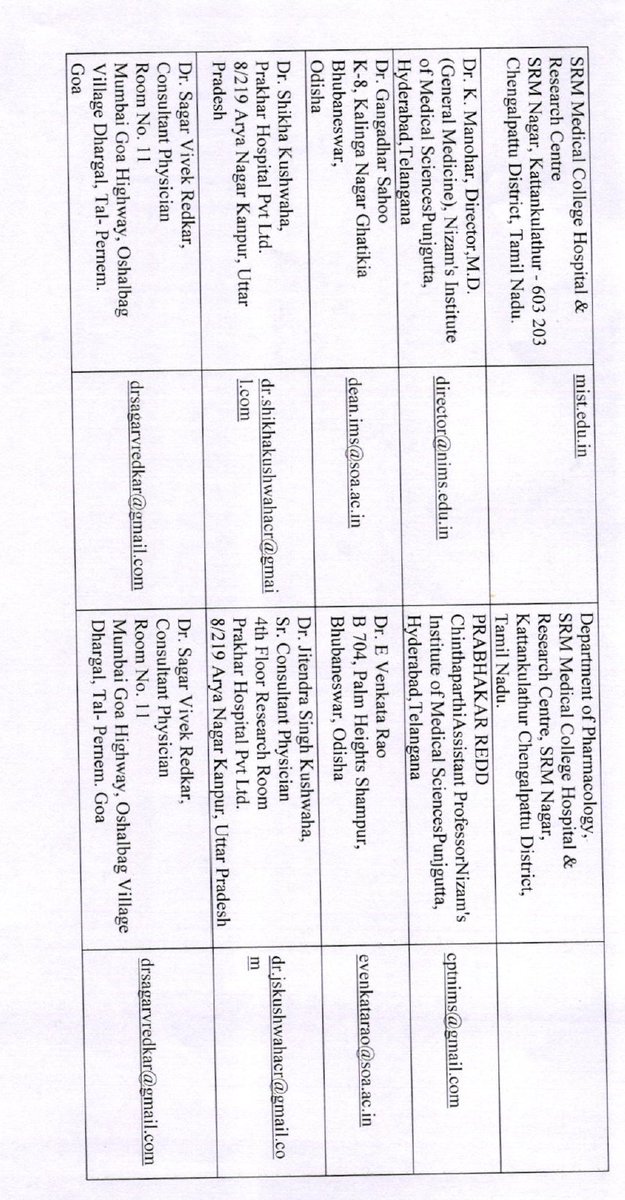
The ICMR DG and some other experts have argued that phase 1 and phase 2 data is enough for restricted emergency use approvals, and that phase 3 efficacy data is not necessarily required. 

Dr T Jacob John also makes an argument in this regard, though also clarifying that there might have been some early safety data from those vaccinated in the phase 3 which is ongoing as well 

BUT if we were going to base a regulatory decision on just phase1 and phase2 data, why did we have to wait till 02nd Jan 2021. That data was available a few weeks earlier, and in fact was considered in the 09th December CDSCO SEC meeting. Why not take the decision then itself? 

why was the SEC continuing to ask for efficacy data on 09th Dec, 30th Dec, 01st Jan and then suddenly decided they did not need it on 02nd Jan?
#transparency #regulation #COVID19 #vaccines
#transparency #regulation #COVID19 #vaccines
• • •
Missing some Tweet in this thread? You can try to
force a refresh