The @CDCgov Advisory Committee on Immunization Practices (ACIP) emergency meeting on the #JNJ vaccine is ongoing. Currently, they're reviewing known data on 8 cases of thrombotic thrombocytopenia. Follow this thread for updates. 1/ #COVID19 #MedTwitter ustream.tv/channel/VWBXKB…
This was the initial @US_FDA announcement yesterday describing the #COVID19Vaccine #JNJ hold. In summary, 6 post-market cases of cerebral venous thrombosis reported as of yesterday (in ~6.8 million doses delivered) prior to the hold. 

The awesome @acweyand excellently summarized the data known as of yesteday from the similar #AstraZenaca adenovirus vector vaccines and background on #CVST! #hemetwitter 3/
https://twitter.com/acweyand/status/1382156548571590657?s=24
The @CDCgov Health Alert Network recommendations:
1⃣ #JNJ vaccine recipients-monitor for headache, weakness, leg pain, shortness of breath and seek medical care if needed
2⃣ #HCWs should report all possible events in #VAERS vaers.hhs.gov/reportevent.ht…
3⃣ PF4 ELISA to screen
4/
1⃣ #JNJ vaccine recipients-monitor for headache, weakness, leg pain, shortness of breath and seek medical care if needed
2⃣ #HCWs should report all possible events in #VAERS vaers.hhs.gov/reportevent.ht…
3⃣ PF4 ELISA to screen
4/

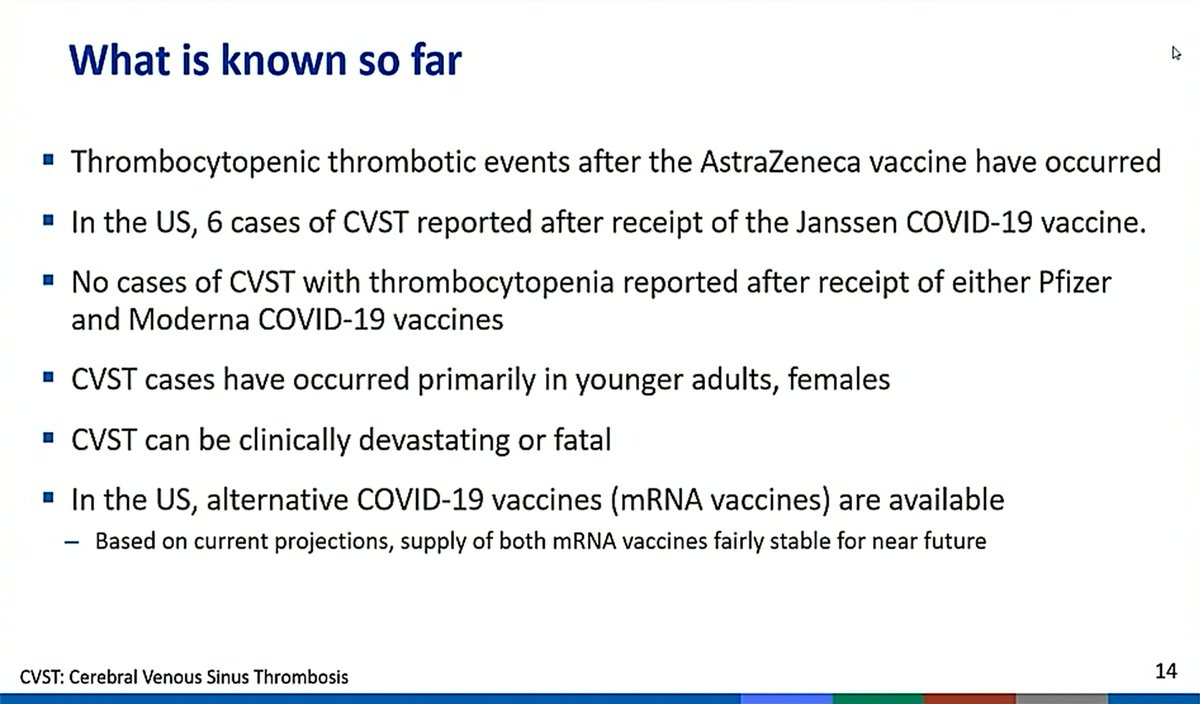
Of the 3 #JNJ vaccine trials (1-dose, 2-dose, SA HCWs): ➡️1 CVST case reported in >340,000 vaccinated patients
⏩The patient had concurrent thrombocytopenia
➡️1 case of CVST in a control arm patient
This frequency isn't all that different than post-trial data ...
5/


⏩The patient had concurrent thrombocytopenia
➡️1 case of CVST in a control arm patient
This frequency isn't all that different than post-trial data ...
5/



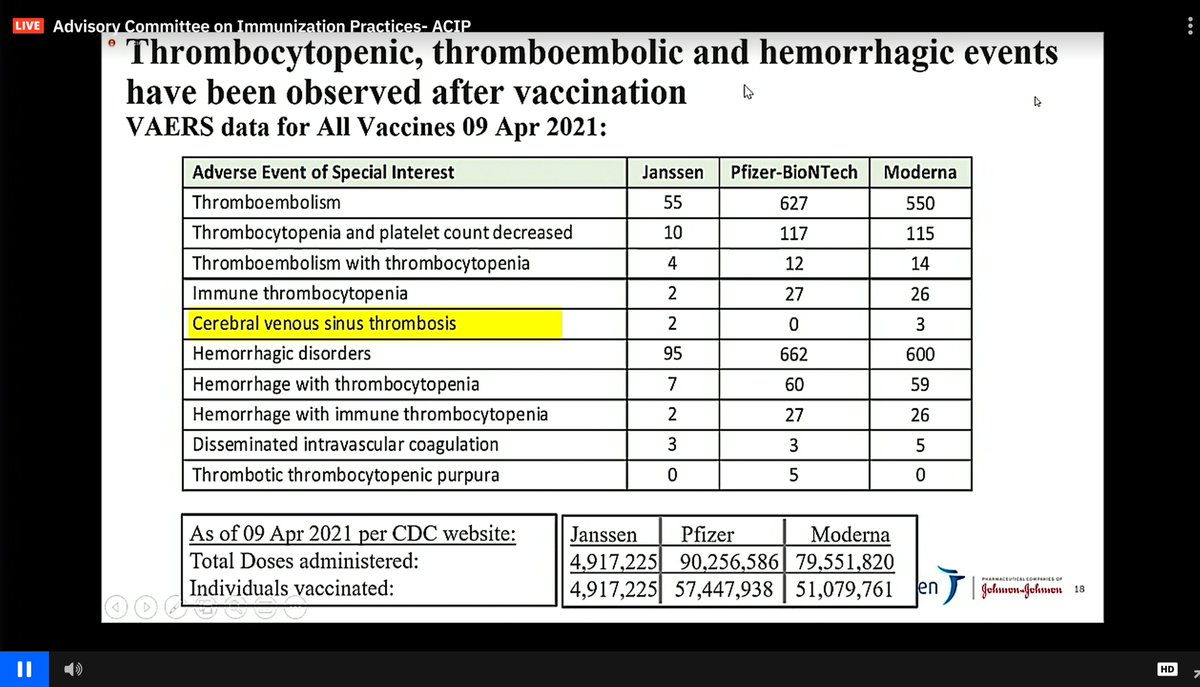
Updated reporting today now has a 7th post-EUA #CVST case reported through @HHSGov #VAERS. We're waiting on more information on this case. Of these 7 cases, 1 death has been reported. 3 patients are still hospitalized (2 in the ICU) and the others have been discharged.
6/
6/

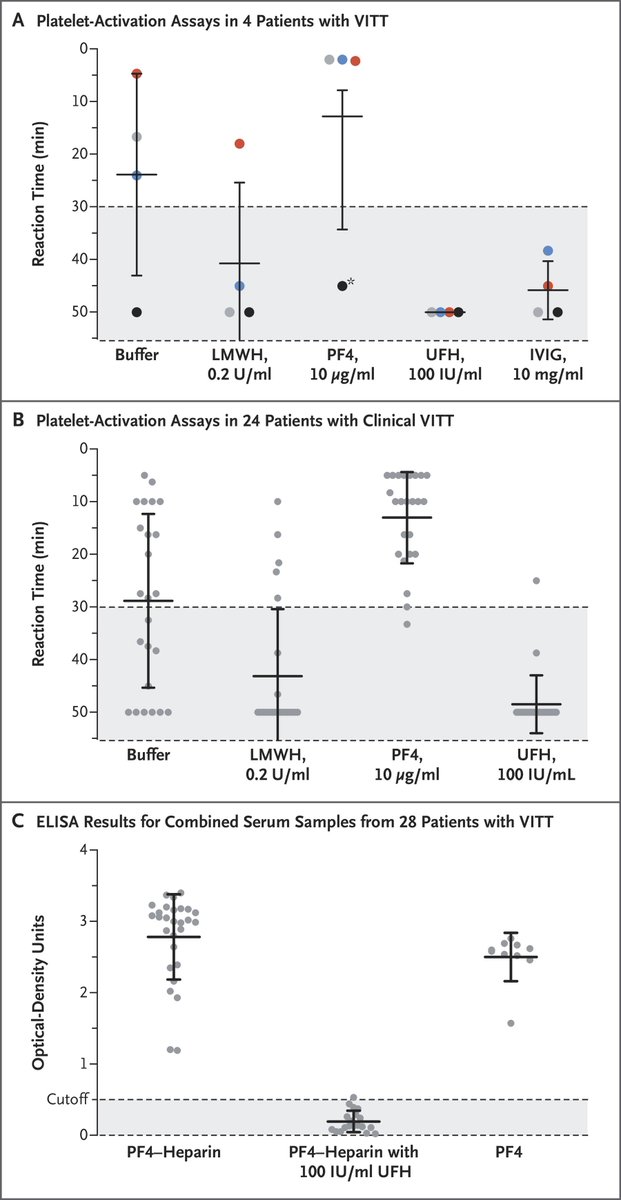
Of the 6 thrombotic thrombocytopenia #CVST cases in the #JNJ vaccine reported as of April 13, 5 of 6 tested positive for PF4 IgG, supporting concerns for a similar mechanism of action to that reported in the #AstraZeneca vaccine in Europe. nejm.org/doi/full/10.10…
7/
7/

Data from last week:
-Only 2 cases of #CVST in the #JNJ vaccine were reported last week, which may explain the rapid change in strategy this week with 5 additional cases identified
-The vast majority of vaccines delivered in the US to this point have been #Pfizer and #moderna
8/
-Only 2 cases of #CVST in the #JNJ vaccine were reported last week, which may explain the rapid change in strategy this week with 5 additional cases identified
-The vast majority of vaccines delivered in the US to this point have been #Pfizer and #moderna
8/
While there are concerns that this #JNJ vaccine "pause" may worsen community #vaccinehesitancy, the fact that this possible rare complication & potential mechanism could be identified out of millions of doses in just over a month shows the @HHSGov #VAERS system works!
9/
9/

Sara Oliver from the @CDCgov ACIP excellently outlines the #JNJ vaccine considerations:
1⃣~3.7 million still in the 2 week window when new #CVST cases might present
2⃣Stable supply of alternative #mRNA vaccines available (currently <5% vaccines delivered in US were #JNJ)
10/

1⃣~3.7 million still in the 2 week window when new #CVST cases might present
2⃣Stable supply of alternative #mRNA vaccines available (currently <5% vaccines delivered in US were #JNJ)
10/

3⃣Primarily in young, female pts post-#JNJ (not in #mRNA vaccines)
4⃣Background population #CVST case rate unknown for a good comparison
5⃣Risk factors for #VITT (thrombotic thrombocytopenia) are unknown currently
6⃣True event rate TBD since more cases may be identified
11/


4⃣Background population #CVST case rate unknown for a good comparison
5⃣Risk factors for #VITT (thrombotic thrombocytopenia) are unknown currently
6⃣True event rate TBD since more cases may be identified
11/


7⃣Policy options range from recommending against #JNJ vaccine to targeted recommendations to recommending the vaccine to all ≥18
8⃣Not enough info yet to make Sex & Age-based recommendations
12/


8⃣Not enough info yet to make Sex & Age-based recommendations
12/



9⃣Complicated risk-benefit considerations between:
➡️Extended pause to collect more information, including data for possible targeted #JNJ vaccine delivery
13/
➡️Extended pause to collect more information, including data for possible targeted #JNJ vaccine delivery
13/

➡️Broad-ranging complications due to delaying:
⏩Opportunity to those who many prefer #JNJ
⏩Access to lower-income regions/countries (e.g., limited freezer capacity for #mRNA vaccines, limited overall international supply of vaccines)
⏩Causing worsened #vaccinehesitancy
14/
⏩Opportunity to those who many prefer #JNJ
⏩Access to lower-income regions/countries (e.g., limited freezer capacity for #mRNA vaccines, limited overall international supply of vaccines)
⏩Causing worsened #vaccinehesitancy
14/

🔟These questions were then posed to the @CDCgov #ACIP and the discussion is ongoing.
One predominant argument so far seems like an extended pause is needed to collect data with the nonmaleficence principle in mind (#DoNoHarm), when we have other safe, effective vaccines.
15/
One predominant argument so far seems like an extended pause is needed to collect data with the nonmaleficence principle in mind (#DoNoHarm), when we have other safe, effective vaccines.
15/

The current speaker is discussing population-level & individual-level risk balance. This cuts to the heart of the issue when there are other viable vaccine options. Obviously, this assumes that future complications won't be discovered (on the production end or side effects).
16/
16/
One of the #JNJ vaccine #CVST cases just published as a Letter in @NEJM during this meeting. This near-real-time information sharing is wild.
17/ nejm.org/doi/full/10.10…
17/ nejm.org/doi/full/10.10…
The last speaker made the point that a non-decision may seem like a decision because of the impact on public trust/perception. The current speaker called for a risk-benefit analysis and it must consider the impact on those with limited access and the impact on at-risk groups.
18/
18/
The current speaker on the @CDCgov #ACIP (Dr. Shah) brought up the point that any extended pause in the #JNJ vaccine should be coupled with a plan for what would need to be seen to make a recommendation.
19/
19/
The @CDCgov Advisory Committee on Immunization Practices closed business with a decision to NOT make an official recommendation (∴ supporting the status quo pause on #JNJ vaccine usage) with plans to reconvene in 7-10 days & review a formal risk-benefit analysis.
#MedTwitter
20/
#MedTwitter
20/
• • •
Missing some Tweet in this thread? You can try to
force a refresh