Thread 🧵👇:
After so many years of work and as a post Epstein-Barr virus infection #MECFS sufferer it makes me really happy that they have put our article on the cover of the August issue of Pathogens journal 👉:mdpi.com/2076-0817/11/8
(1/)
After so many years of work and as a post Epstein-Barr virus infection #MECFS sufferer it makes me really happy that they have put our article on the cover of the August issue of Pathogens journal 👉:mdpi.com/2076-0817/11/8
(1/)
Until recently nobody talked about post-viral syndromes (actually they should be called chronic infectious syndromes) and they left all of us patients isolated both at a health and social level. Unfortunately it took another virus like #SARSCoV2 to show us how viruses are ...(2/)
...capable of developing chronic diseases like #LongCovid . It is curious that for bacterial infections we use antibiotics but for viral infections we leave it to our immune system to manage to control it. This must change. (3/)
We must prevent with antiviral treatments to prevent the development of #chronicdiseases such as Long COVID, ME/CFS, autoimmune diseases and even cancer. It is absurd to think that everyone's immune system can resolve most viral infections. (4/)
Patients who develop diseases associated with these viruses were previously completely #healthy. Having one or another type of MHC-II allele can make you weaker or more resistant to certain viruses. In the case of herpesviruses, it is not the viral particles that ...(5/)
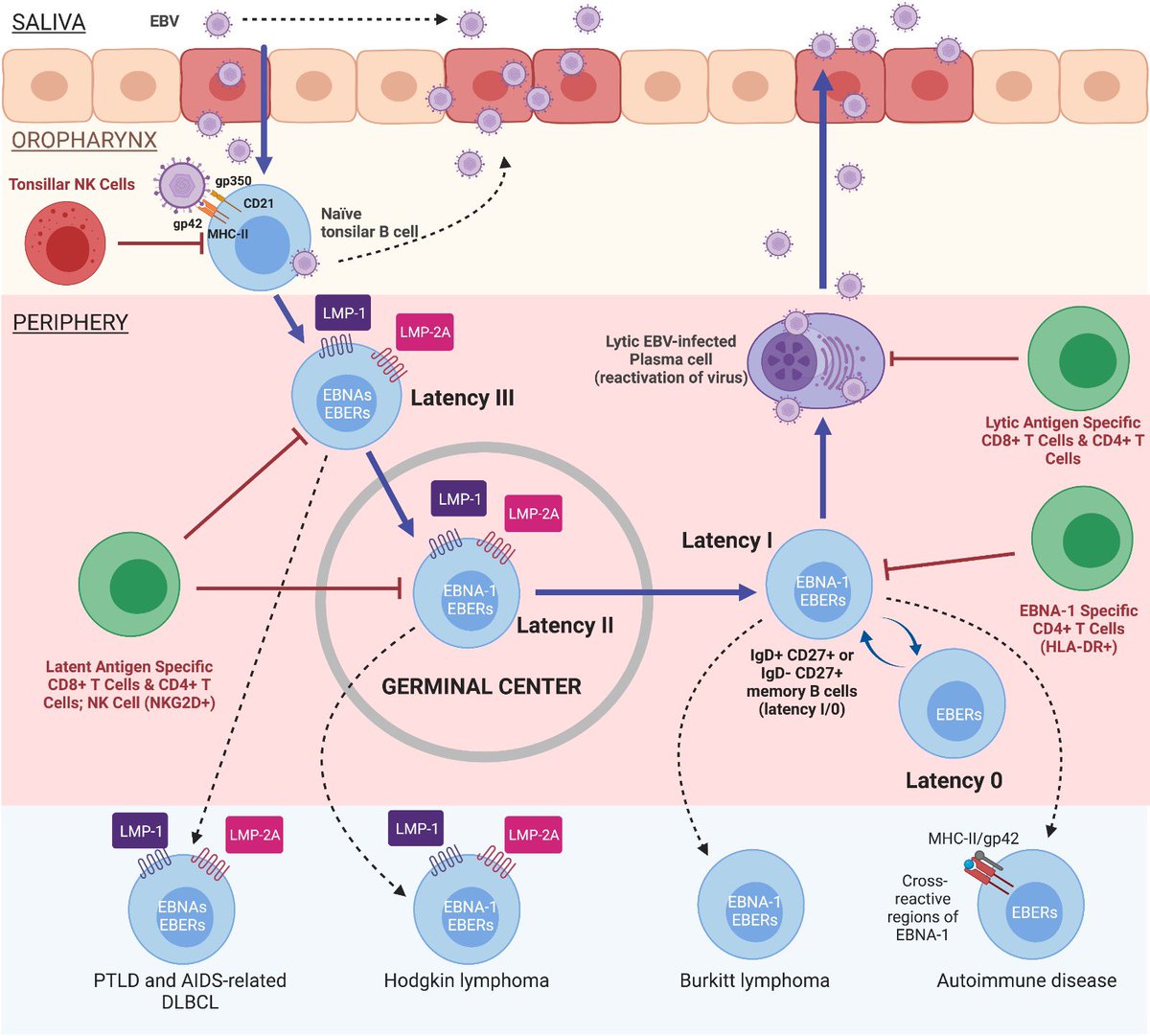
...are the problem, but the latent cells that cause the immune disruption. The Epstein-Barr virus (EBV) is one of the oldest viruses with which humans have coexisted, even since we were apes. Living with this virus has led to the evolution of its evasion mechanisms and...(6/)
...also of our resistance mechanisms (MHC-II alleles).
In the case of EBV, the patient only needs to have one of the "ancestral" alleles (the oldest) of the MHC-II weak against EBV, since one of the main reasons why this virus is related to the MHC-II alleles is ...(7/)
In the case of EBV, the patient only needs to have one of the "ancestral" alleles (the oldest) of the MHC-II weak against EBV, since one of the main reasons why this virus is related to the MHC-II alleles is ...(7/)
...because it infects cells through the interaction of its viral protein gp42 with the cell's MHC-II. In this way it fuses its membrane with that of the cell, forming a new gp42-MHC-II complex that alters antigenic presentation to CD4 T cells,...(8/) mdpi.com/1422-0067/19/1… 

...since MHC-II is essential for the #Immune system to know what is foreign and what is not. By decreasing this antigen presentation to CD4 T cells, an acquired immunodeficiency develops that allows any inflammatory stimulus (other infection) in any tissue to lead to the... (9/)
...formation of EBV-infected ectopic lymphoid aggregates. This, together with the altered immunosurveillance caused by the evasion mechanisms of these cells with EBV latency (increased Th2 and Treg), would lead to increased proliferation of these cells, ...(10/) 

...which would increase the risk of neoplastic transformation or #autoimmune disease in these tissues.
In the case of #LongCovid two things can happen. That patients have other "weak" MHC alleles against that virus or that they are the same weak alleles against #EBV, ... (11/)

In the case of #LongCovid two things can happen. That patients have other "weak" MHC alleles against that virus or that they are the same weak alleles against #EBV, ... (11/)


...allowing #SARSCoV2 infection to cause recruitment of B cells with EBV latency and form these lymphoid aggregates. In other words, EBV is an opportunistic #virus that takes advantage of the infection of other pathogens to settle in different tissues. (12/)
These tissues finally end up with chronic inflammation by activating receptors (TLRs) that continuously detect viral genetic material, causing an increase in the inflammatory and innate response but without the adaptive response being able to... (13/)
...control the #infection completely due to the decrease in the activation of CD4 T cells. This acquired #immunodeficiency also causes reactivation of other latent pathogens such as other #herpesviruses and Parvovirus B19, adding more problems and more #inflammation. (14/)
That is why it is common to have viral reactivations in all EBV-associated diseases.
Attached is the full article if you would like more detailed information (15/): mdpi.com/2076-0817/11/8…
#Immunology #immunity #Covid_19 #viral #cancer #pwME #Lupus #MultipleSclerosis #Health
Attached is the full article if you would like more detailed information (15/): mdpi.com/2076-0817/11/8…
#Immunology #immunity #Covid_19 #viral #cancer #pwME #Lupus #MultipleSclerosis #Health
• • •
Missing some Tweet in this thread? You can try to
force a refresh