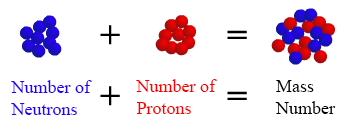
We identify isotopes by their mass number, i.e. cobalt-60 or cobalt-58
Clear as mud? Great, let’s move on 10/
Answering a common question: yes you can own uranium ore 11/
In simple terms, U-235 and U-238 are identical from a chemistry standpoint 22/
✔️number of centrifuges (5060)
✔️the number of cascades (30)
✔️enrichment they can produce (3.67%)
✔️how much they can stockpile (300 kg)