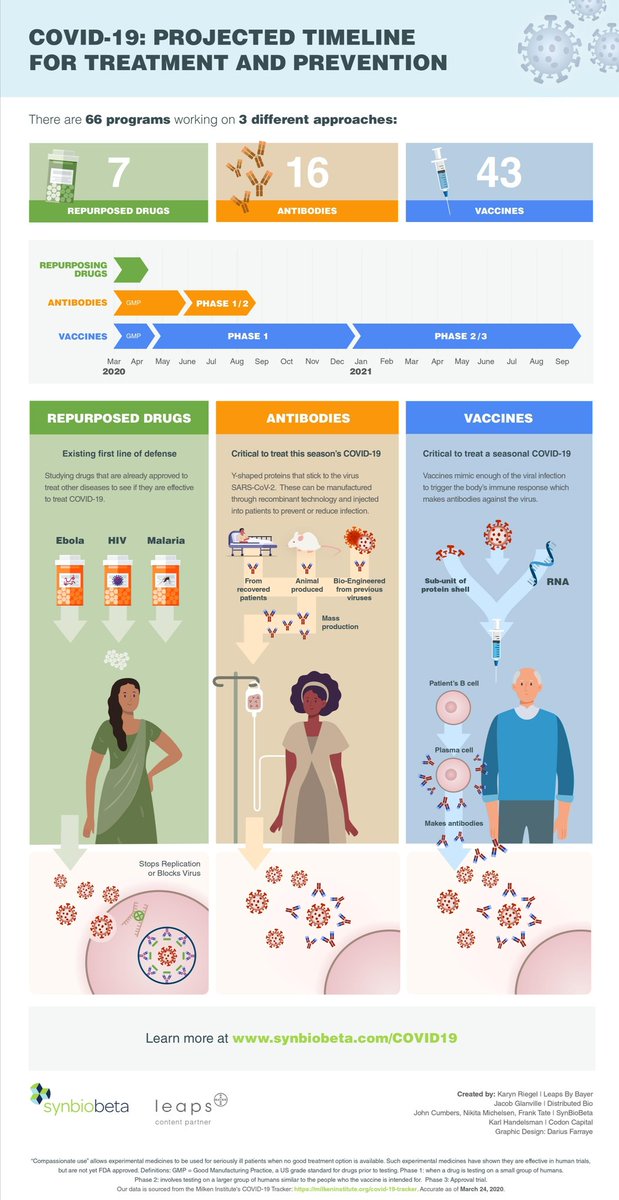
1. phase 1 = few people, assessing *safety*
2. phase 2 = more people, glimpse at response + side effects
3. phase 3 = often multiple trial sites, large scale, looking for efficacy, too
12/n

Preclinical: tests in cells, animals
IND: investigational new drug (application) = what drugmakers submit when they want to start a clinical trial
NDA: new drug application = what they submit to get approval to market a drug
14/n