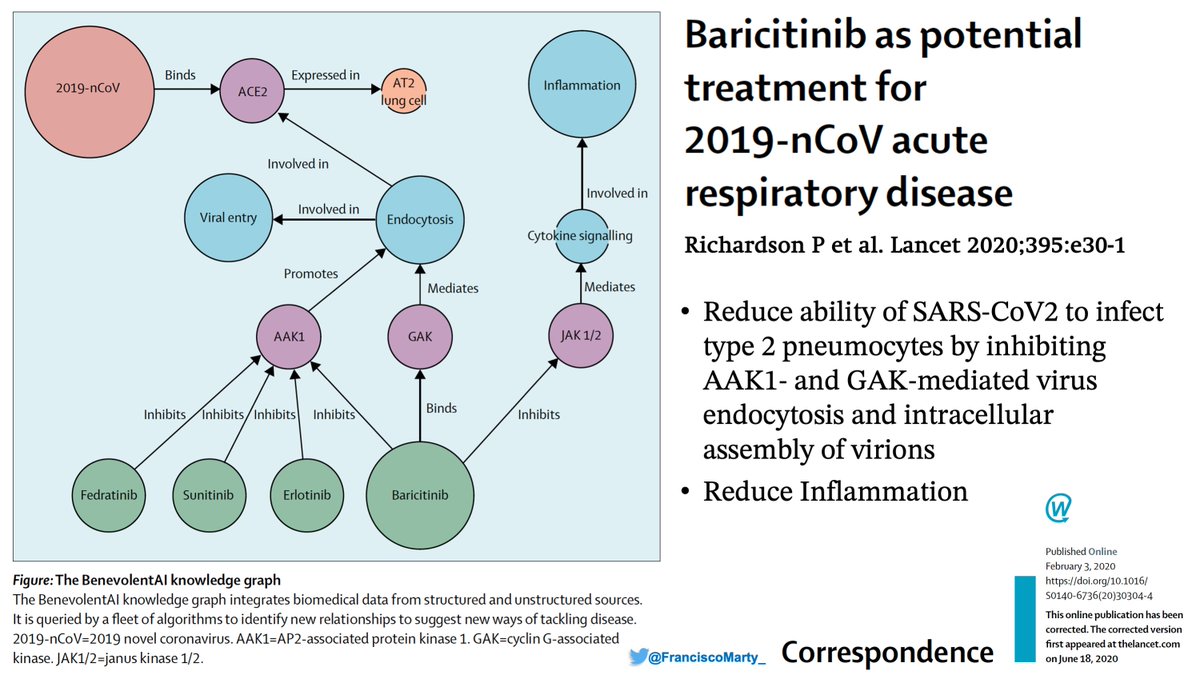
Finally got to analyze & put my thoughts together on the recent #posaconazole vs. #voriconazole phase 3 trial for invasive #aspergillosis published in .@TheLancet a few weeks ago.
Best is to put the major trials of the 3 #VIP triazoles done over the last 20 years in perspective!
Best is to put the major trials of the 3 #VIP triazoles done over the last 20 years in perspective!

So (re)studied the following key trials & appendices
#Posaconazole vs. #Voriconazole: thelancet.com/journals/lance…
#Isavuconazole vs. Vori: thelancet.com/journals/lance…
#AmphotericinB vs. Vori: nejm.org/doi/10.1056/NE…
#TxID #IDTwitter
#Posaconazole vs. #Voriconazole: thelancet.com/journals/lance…
#Isavuconazole vs. Vori: thelancet.com/journals/lance…
#AmphotericinB vs. Vori: nejm.org/doi/10.1056/NE…
#TxID #IDTwitter
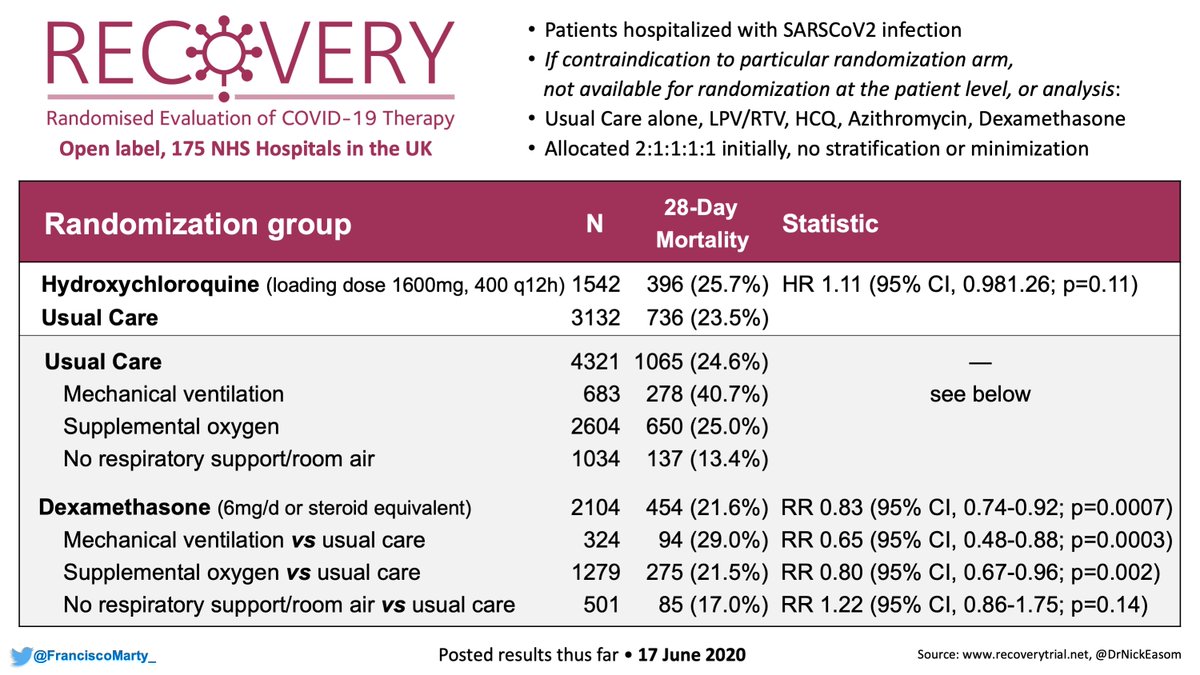
Here is the table with key points of trial design of the #TresAmigos trials, interesting features:
#Vori-Ampho was open label, but with blinded adjudication, including to adverse effects.
All where non-inferiority trials, #vori-amphoB had a clause for claiming superiority.
#Vori-Ampho was open label, but with blinded adjudication, including to adverse effects.
All where non-inferiority trials, #vori-amphoB had a clause for claiming superiority.

#voriconazole dosing strategy was different in the 3 trials
IV vori loading was required in the vori-AmphoB and vori-isa trial, not so in the posa-vori
The posa-vori protocol preferred IV loading, but ~50% of patients loaded orally, using 300mg x 2 doses. A detail to keep in mind
IV vori loading was required in the vori-AmphoB and vori-isa trial, not so in the posa-vori
The posa-vori protocol preferred IV loading, but ~50% of patients loaded orally, using 300mg x 2 doses. A detail to keep in mind
The vori-AmphoB was published before .@CONSORTing standards, so we're missing some info.
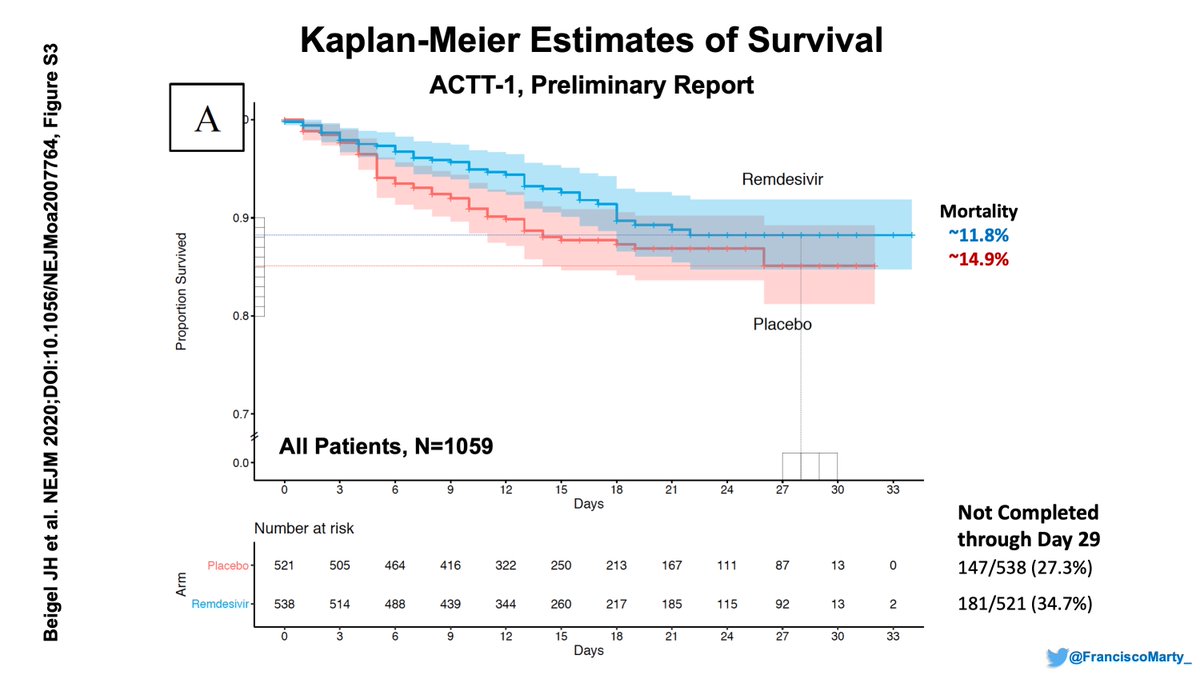
Discontinuation rates due to adverse events were same for vori in both triazole studies, lower in isa, though in the trial, 6% more patients d/c isa for inadequate response (Fig. 1).
Discontinuation rates due to adverse events were same for vori in both triazole studies, lower in isa, though in the trial, 6% more patients d/c isa for inadequate response (Fig. 1).

Here are the core results of the #tresamigos in a table.
Used the 2015 reclassification of the Vori-AmphoB trial here: academic.oup.com/cid/article/60…
As .@davidvanduin has pointed out, we have not improved on overall #mortality outcomes in the 20 years of the VIP #triazole era.
Used the 2015 reclassification of the Vori-AmphoB trial here: academic.oup.com/cid/article/60…
As .@davidvanduin has pointed out, we have not improved on overall #mortality outcomes in the 20 years of the VIP #triazole era.

So on an overall efficacy perspective, no real difference I can put my finger on.
What about safety?
I have summarized the major adverse effects per system using MedDRA as reported in the last 2 trials, putting voriconazole in each trial next to each other to facilitate read.
What about safety?
I have summarized the major adverse effects per system using MedDRA as reported in the last 2 trials, putting voriconazole in each trial next to each other to facilitate read.

One of the things that I felt could be misleading i the paper was the report of "related" AEs in the main text of the posa-vori paper.
As people pointed out, clinicians knew well the adverse effects of vori, so easier to classify AEs as related for vori and make vori look worse.
As people pointed out, clinicians knew well the adverse effects of vori, so easier to classify AEs as related for vori and make vori look worse.
It was a blinded trial after all & lots of investigators had not used #posaconazole before.
The appendix has all posa-vori AE results. There are not good explanations why AEs for respiratory, metabolic, and renal disorders were significantly higher among posa vs. vori patients.
The appendix has all posa-vori AE results. There are not good explanations why AEs for respiratory, metabolic, and renal disorders were significantly higher among posa vs. vori patients.

The posa-vori paper limits its presentation to treatment-related AEs, which I think it is not appropriate. What specific adveres effects led to these increased signals in the #posaconazole arm is not presented and important to better understand the trial.
Some of the adverse effects may be related to the #posaconazole induced #pseudohyperaldosteronism described by .@GRThompsonMD's team here: academic.oup.com/cid/article-ab… 

So in terms of overall safety, there was no adverse event group were posa did significantly better than vori, in contrast, isa did better than vori in several AE domains as shown. So looking at the data to data, isa seems to be the safest of the 3 amigos for IA. 
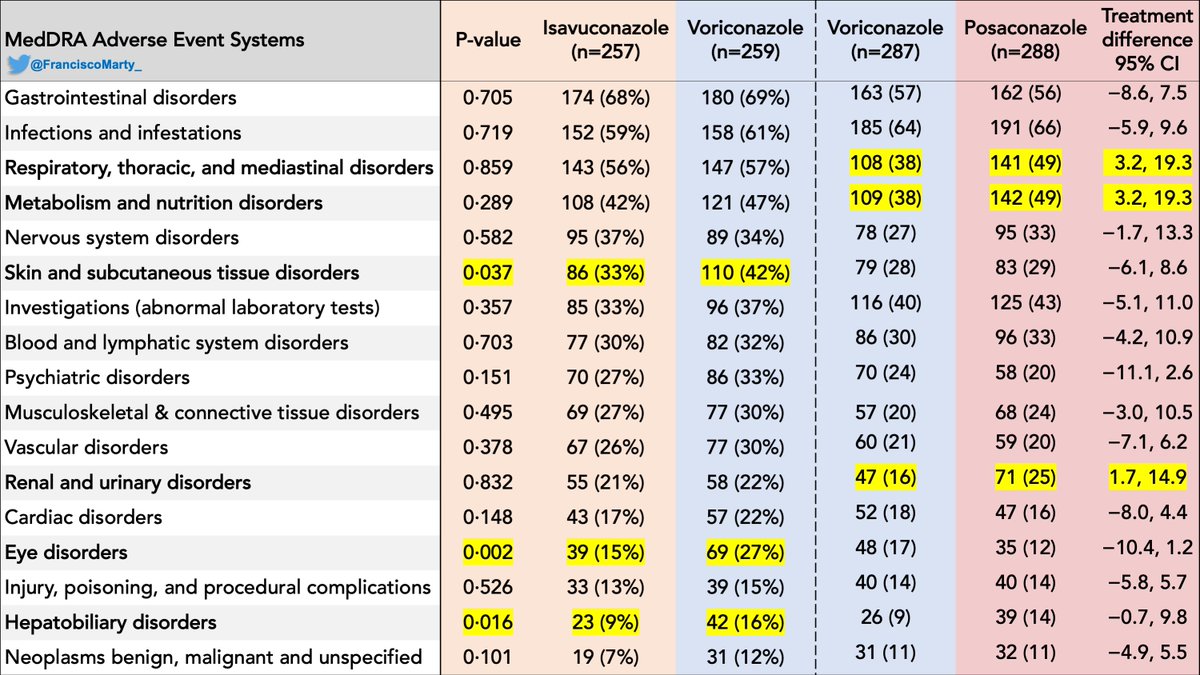
A disturbing finding is that patients who had probable or proven invasive aspergillosis had identical mortality with either vori or posa, but those who did not (empirical therapy, unclassifiable) did worse on vori, and drove the small difference reported. 



it points out to the importance of avoiding empiricism in the ICH, making a firm IA diagnosis, how many delayed treatments for other conditions occurred?
Also reminded me of the old Vori vs. LAmB paper where vori did not meet non-inferiority criteria and was never approved x F+N.

Also reminded me of the old Vori vs. LAmB paper where vori did not meet non-inferiority criteria and was never approved x F+N.


The paper came out together with a letter from the .@US_FDA explaining the proper analysis of the trial and what to consider moving forward. 

So how to best use the 3 triazole amigos against invasive aspergillosis?
Efficacy is really similar between the 3 with overall, safety is a bit better with isa than posa given the overall results without drug level monitoring, done in none of the 3.
Efficacy is really similar between the 3 with overall, safety is a bit better with isa than posa given the overall results without drug level monitoring, done in none of the 3.

So drug level monitoring can improve the safety of the treatments, but needs to be assessed in terms of severity of disease, as sicker patients likely got IV longer and may have been overexposed to vori or posa.
Awaiting the secondary PK paper on the posa-vori study to understand
Awaiting the secondary PK paper on the posa-vori study to understand
Another caveat is that, the posaconazole formulations used (IV in cyclodextrine, ER tablets) are different from the oral suspension, not available in most of the world even today.
And then we have the issue of cost toxicity. Here is my updated table of US pricing as of today
And then we have the issue of cost toxicity. Here is my updated table of US pricing as of today
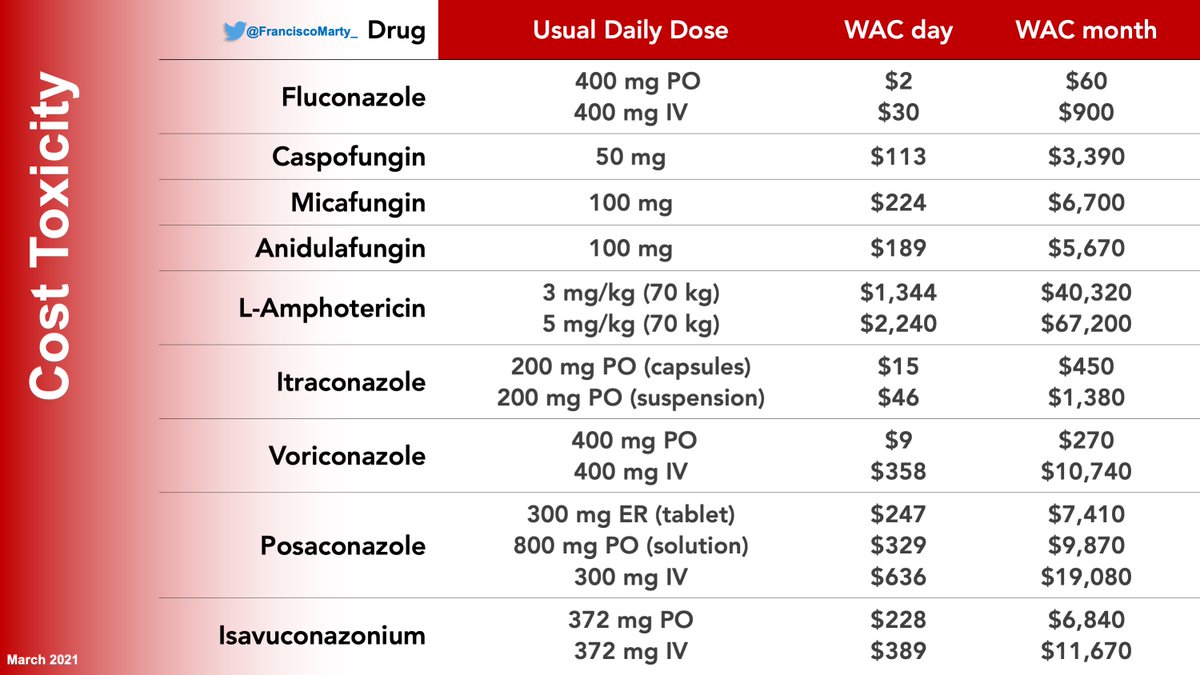
Vori being so inexpensive, will remain the go-to antifungal for aspergillosis until the other amigos become generic, the competition has brough posa price down, isa price up.
Here is a summary slide I did for a lecture in #Brazil where Flex cars use either gasoline or ethanol.
Here is a summary slide I did for a lecture in #Brazil where Flex cars use either gasoline or ethanol.

And that is what we are doing these days: #voriconazole remains the first line drug for IA. For patients with obvious contraindications (liver enzymes, encephalopathy, QTc prolongation, important drug-drug interactions), using #isavuconazole or #posaconazole are good alternatives
• • •
Missing some Tweet in this thread? You can try to
force a refresh