It took a while to digest the details of over 600 pages, but here is my review and comparative analysis of the #ACTT2 trial that compared #baricitinib vs. #placebo among patients receiving #remdesivir for #COVID19!
Some very interesting insights, I think!
Sit briefly and enjoy!
Some very interesting insights, I think!
Sit briefly and enjoy!

Will be mainly comparing #ACTT2 to #ACTT1, but also will get some insights and questions that come up when comparing #ACTT2 to the #dexamethasone #RecoveryTrial 

The idea of using #baricitinib (aka Bari) for #COVID19 is in this commentary in .@TheLancet from Feb. when Peter Richardson & Co working .@benevolent_ai .@ucl searched drugs and noted that #bari could block inflammation & viral endocytosis.
link: thelancet.com/journals/lance…
link: thelancet.com/journals/lance…
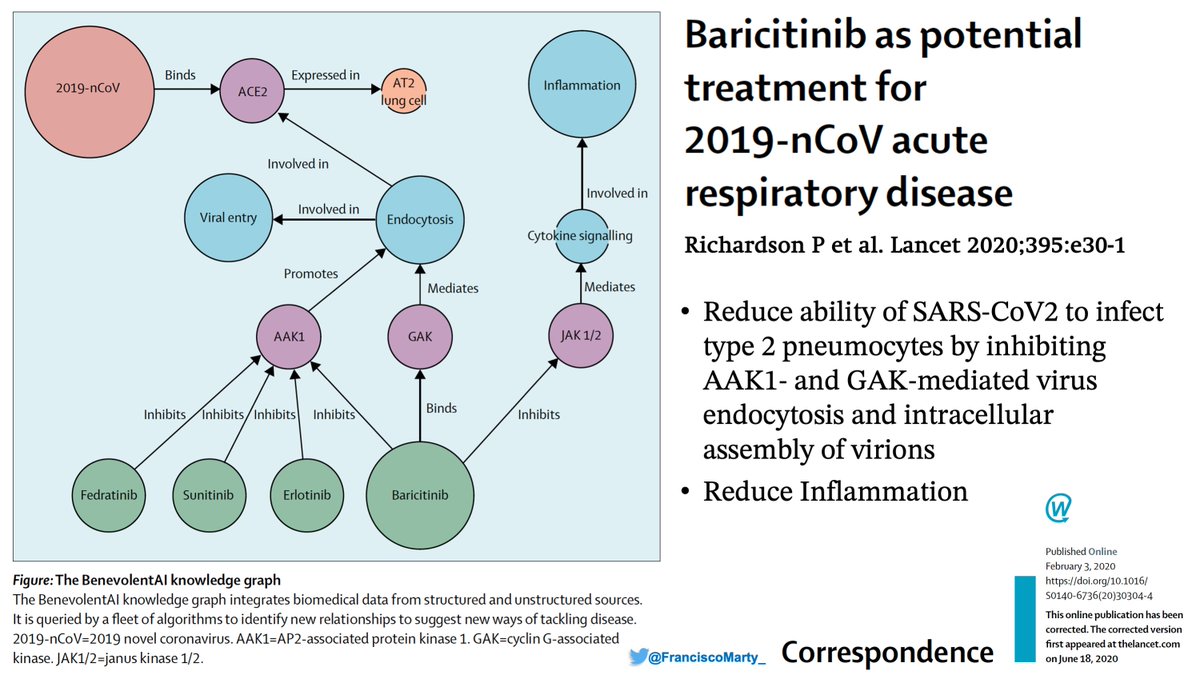
At .@EmoryUniversity .@GradyHealth & .@AtlantaVAMC, my esteemed friend and colleague .@vmarconi2 and his team (.@Boghuma .@AneeshMehtaMD, others) tried it out and they thought there may be a clinical signal...
They proposed to try Bari to .@NIAIDNews
academic.oup.com/cid/advance-ar…

They proposed to try Bari to .@NIAIDNews
academic.oup.com/cid/advance-ar…


As you can see in the original protocol for #ACTT2, before the #DSMB for #ACTT1 stopped the #remdesivir vs. placebo trial, the idea was to do a factorial design... 



Given the #ACTT1 preliminary results, the factorial design was dropped and the decision was to proceed with #bari vs. placebo, everyone getting #remdesivir.
Interesting highlight that plays out later: severe #Covid19 disease defined as high-flow O2 or being on a ventilator
Interesting highlight that plays out later: severe #Covid19 disease defined as high-flow O2 or being on a ventilator
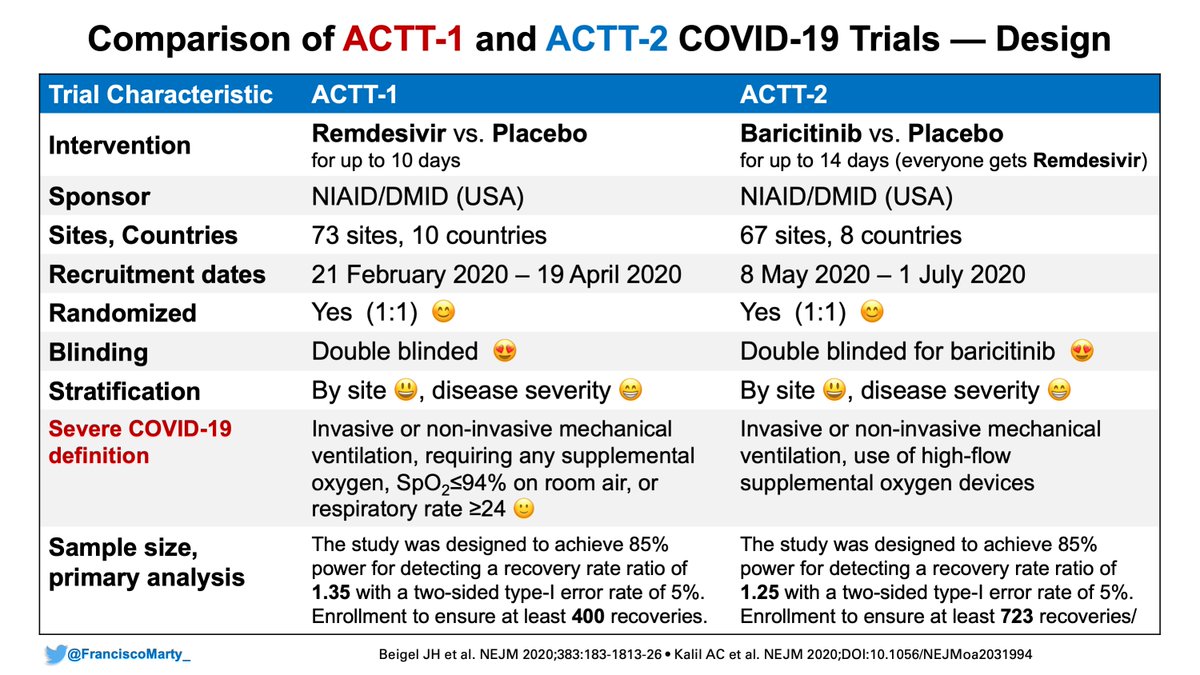
By adding #baricitinib to the protocol (and involving .@LillyPad), many more exclusionary criteria were added to the eligibility, which would exclude a more or less high-risk patients depending on the participating centers (cancer, autoimmune diseases) allowed in #ACTT1. 

Several other patient-level stopping rules that would exclude patients or be taken off study early. Something to keep in mind in the comparisons of recovery and mortality.
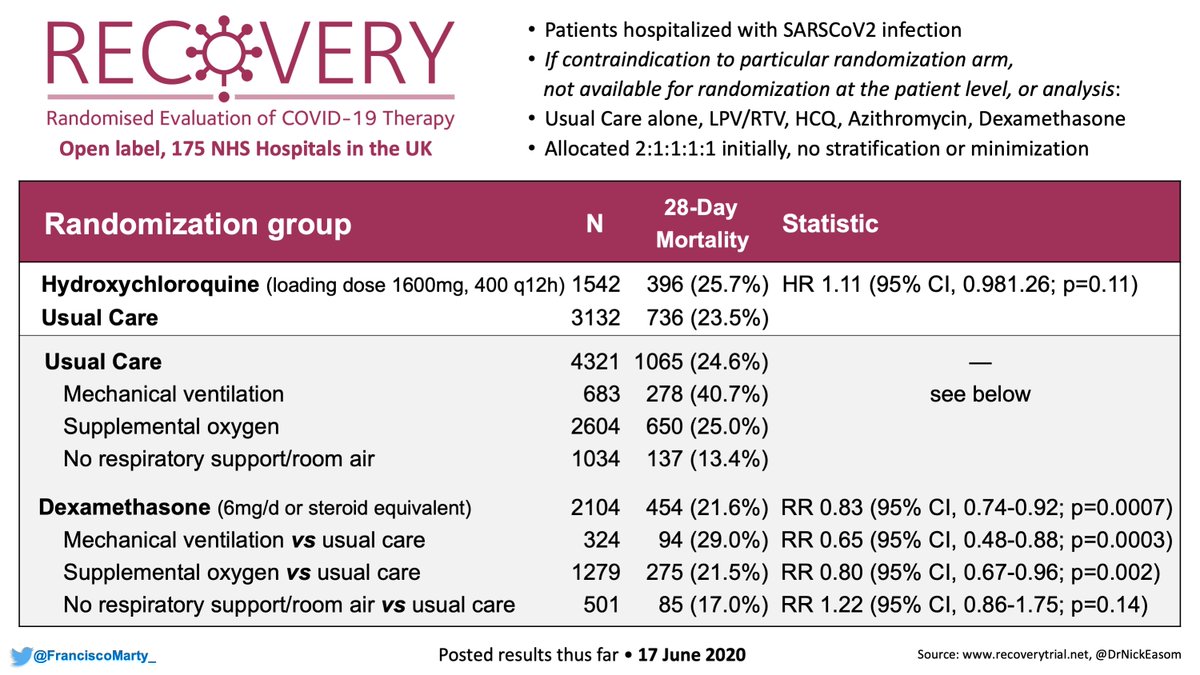
Also no steroids allowed in General:
- Recovery Dex announcement June 16
- ACTT2 closed enrollment July 1
Also no steroids allowed in General:
- Recovery Dex announcement June 16
- ACTT2 closed enrollment July 1

In terms of baseline, #ACTT2 had the first centenarian I've ever encountered in an ID trial!
Lot's of #LatinX (pandemic wave had moved South), and already a shift from #ACTT1's lessons to go for low-flow O2 requiring patients, and less for patients on mechanical ventilation.

Lot's of #LatinX (pandemic wave had moved South), and already a shift from #ACTT1's lessons to go for low-flow O2 requiring patients, and less for patients on mechanical ventilation.


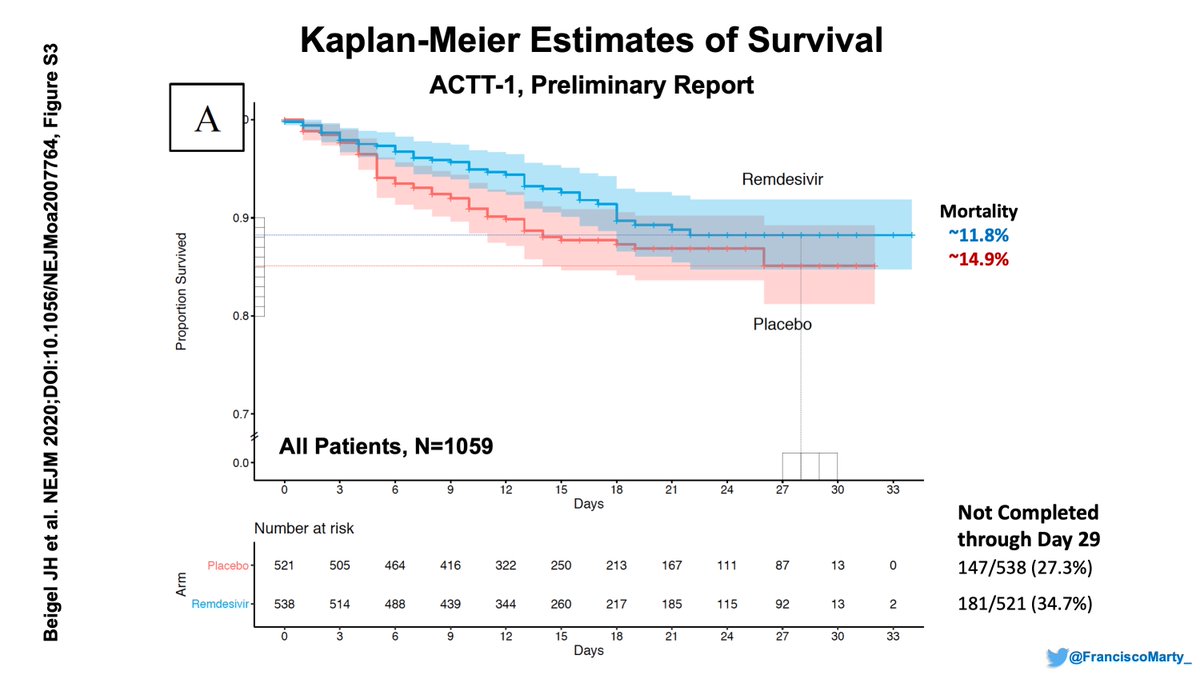
And just to summarize the overall result for #ACTT1, a whooping 5 day absolute decrease in time to recovery (from 15 to 10 d) with #remdesivir, 3.8% lower mortality overall, from (15.2 to 11.4), driven by the low-dose O2 group.
It felt like a kickoff Recovery touchdown!

It felt like a kickoff Recovery touchdown!


#ACTT2's #baricitinib shaved only 1 day overall (from 8 to 7 days), with a #remdesivir/placebo median time down by 2 days on this new trial set.
Felt relieved for .@vmarconi2 bet, but felt more like a dive through the line at the 1-yard touchdown.
Let's dive into more details

Felt relieved for .@vmarconi2 bet, but felt more like a dive through the line at the 1-yard touchdown.
Let's dive into more details


An odd point (free pass by the editors?) is that the primary analysis p-value is from a stratification criteria post-hoc correction (No or low-flow O2 vs. high-flow O2 or mech. ventilation). 0.047 still significant for 1ry endpoint, but I guess feels like the .@Notawful pumpkin. 



When you look at the prespecified analyses by .@WHO ordinal scale, you see no benefit of #baricitinib for people on ventilators or not on O2, but a nice benefit in those on high or low-flow O2, which were the majority in the trial. 







So for those who don't mind the #WHO ordinal scale, the trial did meet their primary endpoint and we now have a large, randomized AND placebo-controlled trial pointing that a anti-inflammatory strategy with #baricitinib is important to improve outcomes in #COVID19 patients. 

Lets look at #mortality to refine our assessments!
Overall, mortality was 2.7% lower in the #baricitinib arm compared to placebo, but the placebo + #remdesivir arm mortality was 7.8% compared to 11.4% in #ACTT1. Part of the difference may be due less patients on the vent...

Overall, mortality was 2.7% lower in the #baricitinib arm compared to placebo, but the placebo + #remdesivir arm mortality was 7.8% compared to 11.4% in #ACTT1. Part of the difference may be due less patients on the vent...


Some fascinating KM curves from the appendices.
- No deaths for No O2 group vs. >4% in ACTT1
- No effect of #baricitinib in patients on vents.
- striking difference in high-flow with a much lower mortality even in the #remdesivir-placebo compared to #ACTT1



- No deaths for No O2 group vs. >4% in ACTT1
- No effect of #baricitinib in patients on vents.
- striking difference in high-flow with a much lower mortality even in the #remdesivir-placebo compared to #ACTT1




Here is a table summarizing the mortalities for #ACTT1 and #ACTT2.
- interesting to see the similar mortality for low-flow O2 patients in both trials besides the observations above.
There were some things, not necessarily fixating on mortality that I missed from ACTT2 and ACTT1

- interesting to see the similar mortality for low-flow O2 patients in both trials besides the observations above.
There were some things, not necessarily fixating on mortality that I missed from ACTT2 and ACTT1


One was missing data for Day 28 mortality, 13-14% is a lot for a mostly US-based trial where you can call (or even call the police) to make sure someone is still alive. The proportion is not mentioned in #ACTT1.
Sensitivity analysis should have been missing=death, but not done.
Sensitivity analysis should have been missing=death, but not done.

Another interesting feature is that the distribution of corticosteroid use was higher only in the #remdesivir + placebo group that was on high-flow O2 at baseline, so the benefit occurred despite this imbalance. 

A pre-specified analysis in the protocol, the SAPs, and the #ACTT2 paper, was an analysis based on quartiles of duration of symptoms, which were very illustrative in the #ACTT1 final report. #MIA! 


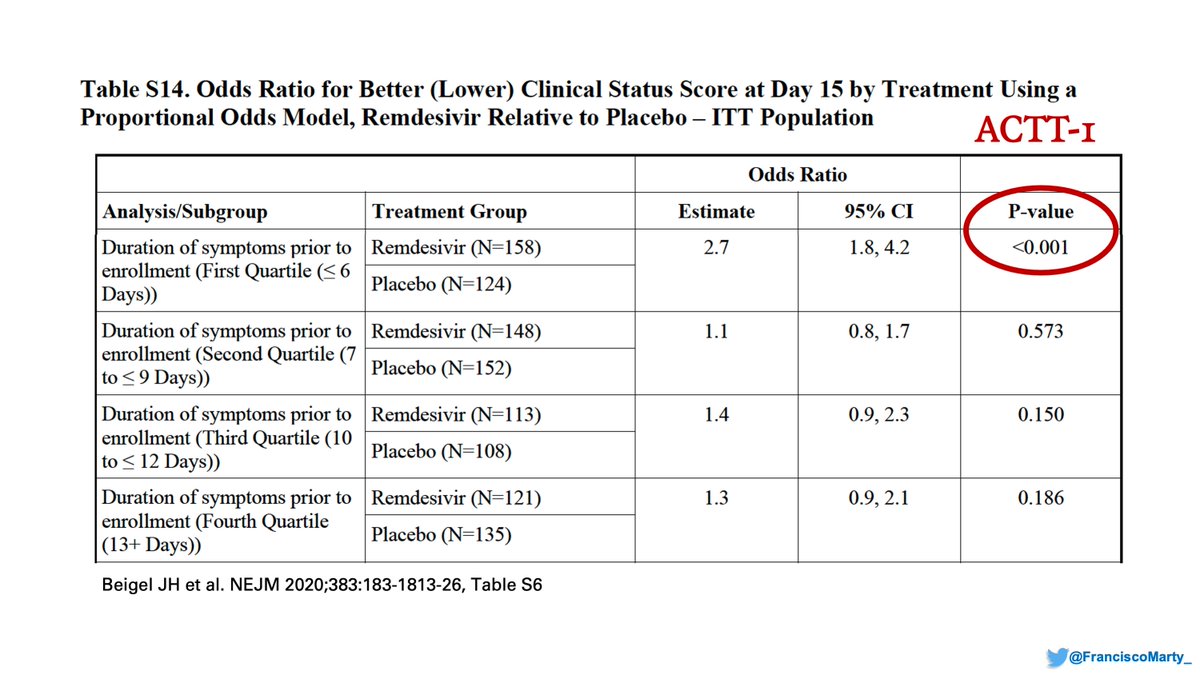
Very relevant because the median days of symptoms went down from 10 in Wang/#Wuhan, to 9 in #ACTT1 to 8 in #ACTT2, with a first quartile that went from 6 to 5 days in ACTT2. If earlier is better, would be good to see the analysis as it can explain some of the lower mortality 

#subgroups? Forrest plots from #ACTT2 & #RecoveryTrial with some interesting comparisons!
For #dexamethasone and #baricitib later was better, but can't compare directly
Middle aged bari patients benefited compared to <70 dex ones
Men benefit from antiinflammatories again...

For #dexamethasone and #baricitib later was better, but can't compare directly
Middle aged bari patients benefited compared to <70 dex ones
Men benefit from antiinflammatories again...


What do I take from this?
1. We now have large blinded data demonstrating anti-inflammatory treatments for #COVID19 are beneficial for patients who are sick enough to require O2 supplementation.
2. ACTT4 will comparedbari and dex blindly: clinicaltrials.gov/ct2/show/NCT04…
1. We now have large blinded data demonstrating anti-inflammatory treatments for #COVID19 are beneficial for patients who are sick enough to require O2 supplementation.
2. ACTT4 will comparedbari and dex blindly: clinicaltrials.gov/ct2/show/NCT04…
3. We have a big discrepancy for patients on ventilators with #RecoveryTrial demonstrating a large benefit for dex and #ACTT2 demonstrating no benefit.
This should have prompted confirming the issue in mechanically ventilated patients, something not in #ACTT4 protocol, would add.
This should have prompted confirming the issue in mechanically ventilated patients, something not in #ACTT4 protocol, would add.
4. Say NO to long-#COVID19, keep Covid short!
- Treat patients earlier with #remdesivir, don't wait for them to worsen
~35-50% of patients on low-flow or No O2 can be discharged before day 5 (recovery graphs), no need to complete 5 days of #remesivir.
Happy Solstice, Confluence!
- Treat patients earlier with #remdesivir, don't wait for them to worsen
~35-50% of patients on low-flow or No O2 can be discharged before day 5 (recovery graphs), no need to complete 5 days of #remesivir.
Happy Solstice, Confluence!

• • •
Missing some Tweet in this thread? You can try to
force a refresh