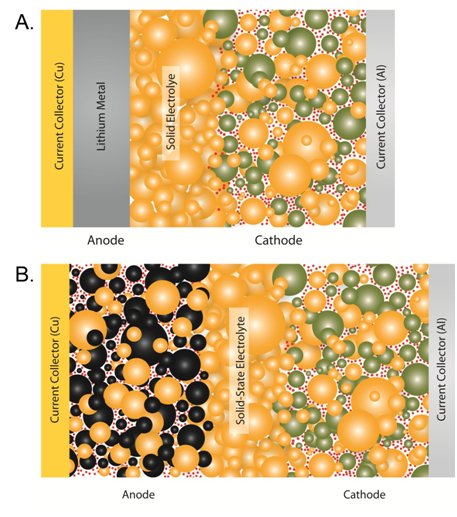
Well, here comes another thread which will hopefully shed some light on this...
But there are also more fundamental problems which are proving tricky to solve...
To be honest, it is very hard to know what is coming. Many people in the industry are in the dark too - they haven't seen any prototypes, and some are skeptical that companies claiming to make solid-state batteries can do so.