Fair warning this isn't going to be short.
jasn.asnjournals.org/content/26/8/2…
*foreshadowing*
An evidence base that is derived primarily from one intervention type simply will not do.
14/21 trials were of RAAS inhibition
7/21 non-RAAS based interventions (2 intensive BP, 2 dietary protein restriction, 2 lipid lowering, 1 glycosaminoglycans)
That 1 trial was of strict BP control in children with CKD.
nejm.org/doi/full/10.10…
"A 30% reduction in albuminuria on top of guideline-recommended care seems necessary to confer a realistically detectable renoprotective treatment effect"
Even one other intervention that had a large enough effect on the surrogate to show benefit?
One intervention does not validate a surrogate.
This is the relationship one intervention (ACEI/ARB) has between albuminuria lowering and ESRD ergo ALL interventions must have this same relationship!
I don't buy it.
40-50% lowering of albuminuria! These must have improved clinical outcomes given their impressive effect on this great surrogate!
The ASCEND trial:
jasn.asnjournals.org/content/21/3/5…
Over 40% albuminuria lowering! Stopped early due to increased fluid overload/CHF.
There is no surrogate for harm.
ncbi.nlm.nih.gov/pubmed/29405626
Over 3600 patients. Average eGFR ~40. Average UACR ~800 mg/g.
~50% albuminuria lowering in the nearly 2700 responders.
ncbi.nlm.nih.gov/pubmed/29604160
news.abbvie.com/news/media-sta…
Full results expected to be published later this year.
nejm.org/doi/full/10.10…
thelancet.com/journals/landi…
Renoprotective effect was in no way modified by albuminuria status. Albuminuria lowering was completely irrelevant to the clinical benefit.
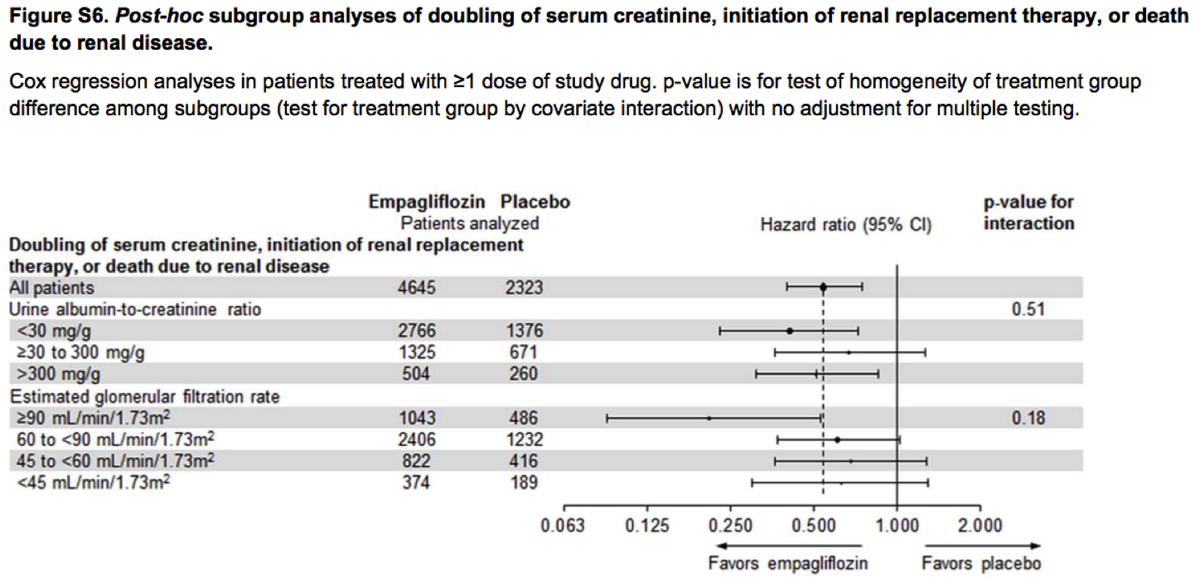
We do not have the intervention diversity necessary to accept albuminuria lowering as a surrogate outcome.
We have examples of interventions that lower proteinuria that can cause harm including dual RAAS blockade (ONTARGET, VA NEPHRON-D) as well as avosentan,....
ncbi.nlm.nih.gov/pubmed/28810023