Why do we use Vitamin C (ascorbic acid) to promote iron absorption?
This question will lead us down fascinating historical paths and eventually provide an explanation to the recently posted @CPSolvers case.
Be sure to listen to the episode first!
clinicalproblemsolving.com/2019/04/18/epi…
Let’s start with a question. What is it about ascorbic acid that promotes absorption of ingested iron?
In order to understand the answer (ascorbic acid REDUCES iron and CHELATES iron), we must first review iron absorption.
Recall that there are two forms of ingested iron: heme and nonheme.
ncbi.nlm.nih.gov/pubmed/23917168
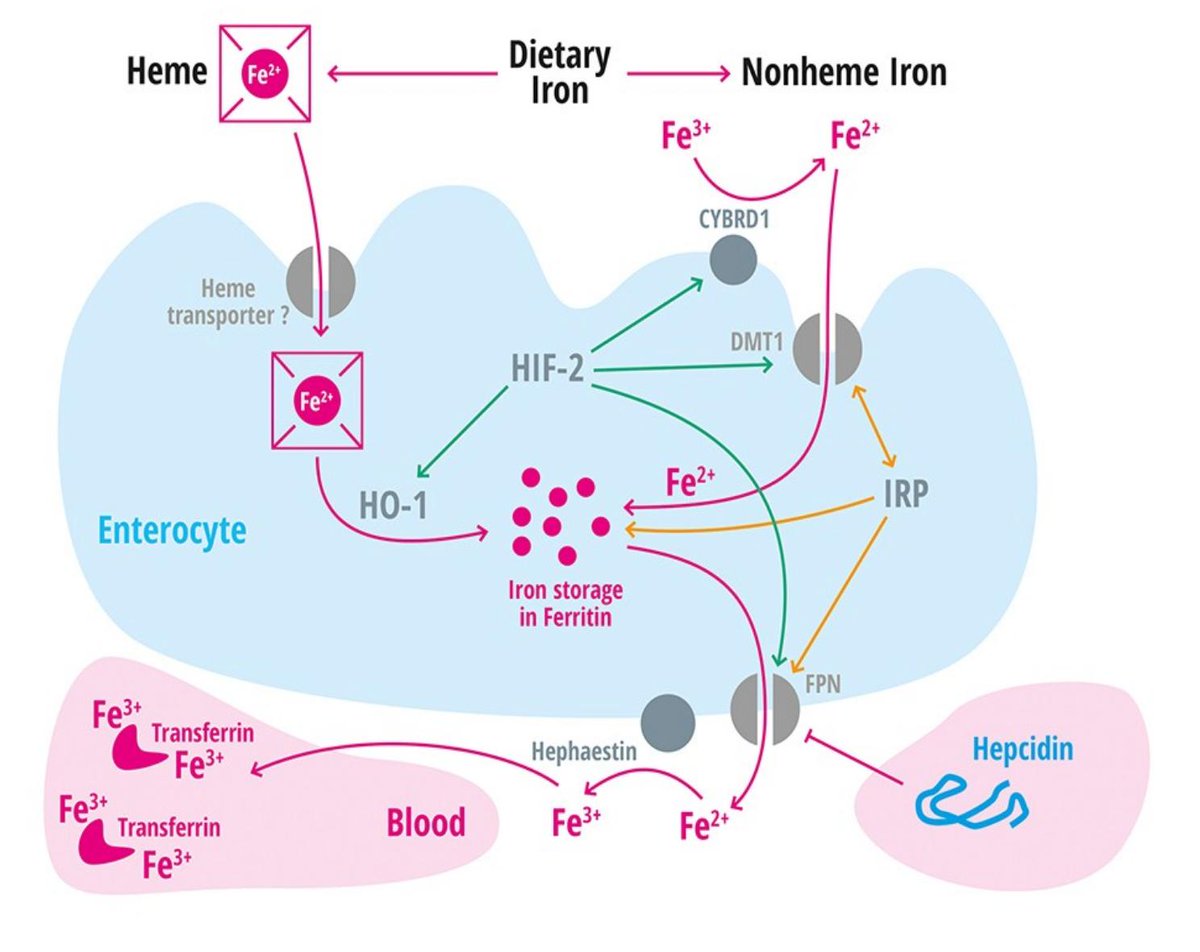
Heme iron is:
• Complexed to heme
• Found in meats
• In ferrous (Fe²⁺) form
• Well absorbed
Nonheme iron is:
• Found in vegetables, cereals, tea, etc
• Typically in ferric (Fe³⁺) form, which is notable because...
• Fe³⁺ is NOT well absorbed
Although heme iron is only 10-15% of the normal oral intake, it contributes 40% of that which is absorbed given that it is more easily absorbed.
This thread will concentrate on nonheme iron as its absorption is modifiable.
ncbi.nlm.nih.gov/pubmed/20200263
Some background:
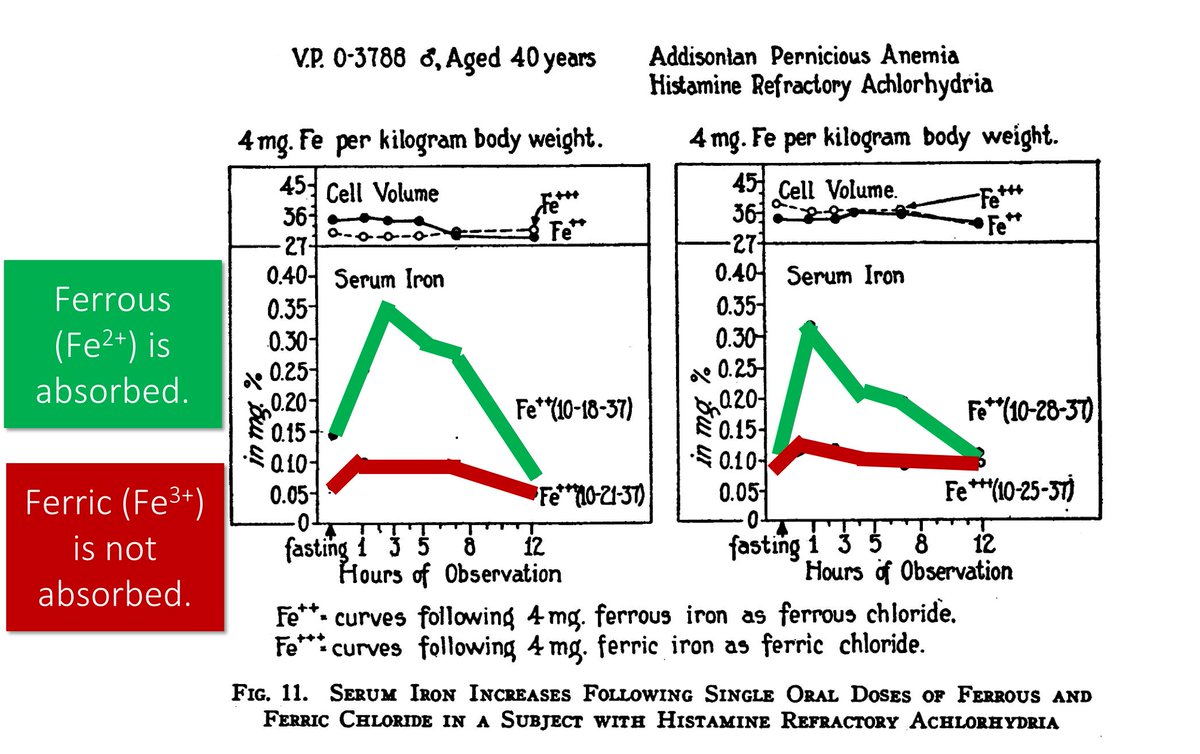
In 1939 it was shown that Fe²⁺ iron can be absorbed at a low OR high pH.
But, in a patient with achlorhydria (i.e., low/no gastric acid production), Fe³⁺ did not absorb. Gastric acid must be doing something.
ncbi.nlm.nih.gov/pubmed/16694687
Gastric acid does a lot. It:
• Releases Fe³⁺ from complexes in food
• Reduces insoluble Fe³⁺ to soluble Fe²⁺, which is more easily absorbed
• Promotes the formation of chelates that also promote absorption
And: Fe³⁺ is more soluble at lower pH.
ncbi.nlm.nih.gov/pubmed/12626689
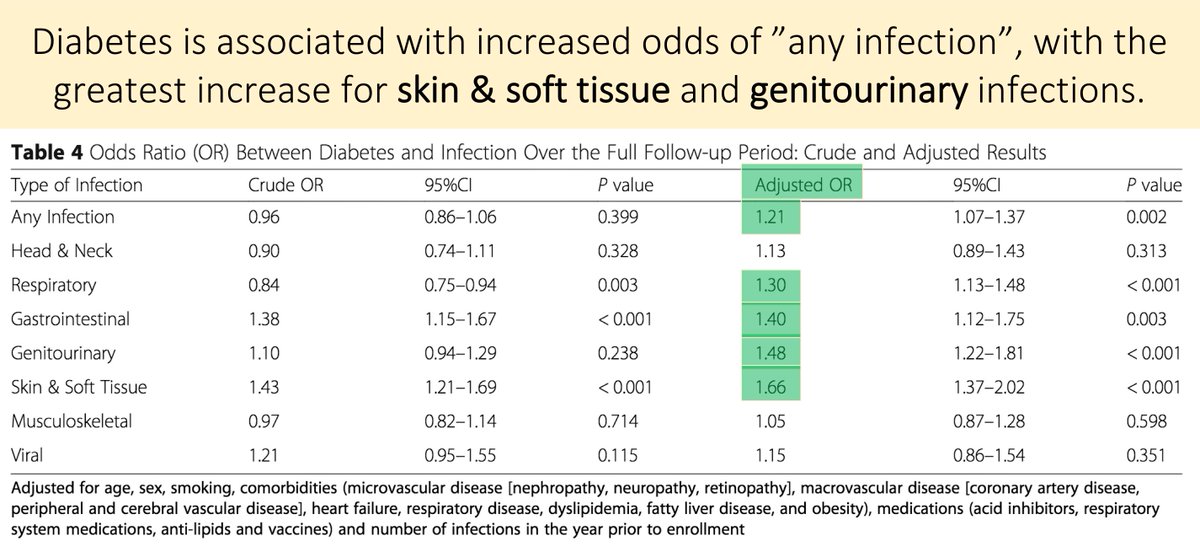
One result: in patients taking chronic acid suppressive therapy (e.g., PPIs), the lack of gastric acid secretion may decrease iron absorption.
This is exactly what was suspected in the @CPSolvers case linked in the first tweet. If you haven't already, go listen!
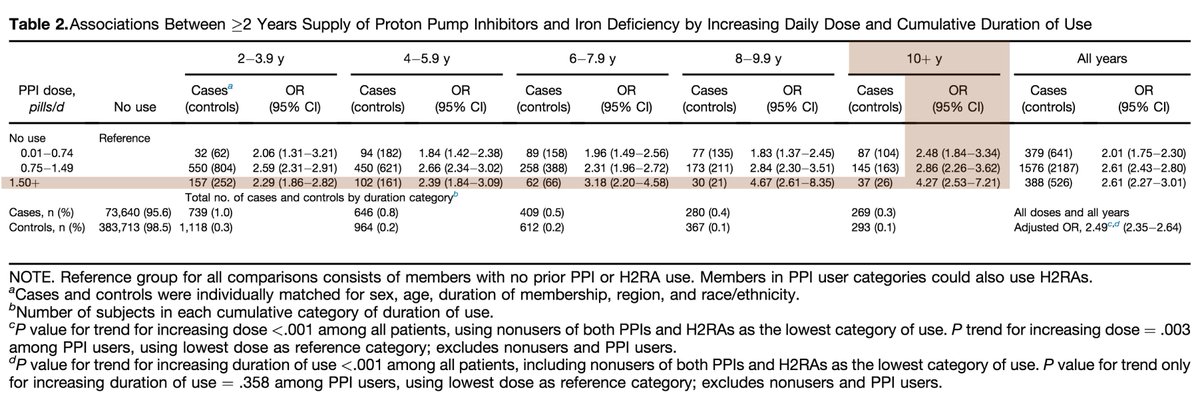
Beyond this amazing @CPSolvers case, there is data suggesting that the risk of decreased iron absorption with PPI therapy is not just theoretical.
One study found an adjusted OR of 4.27 if you took >1.5 pills/day for at least 10 years.
ncbi.nlm.nih.gov/pubmed/27890768
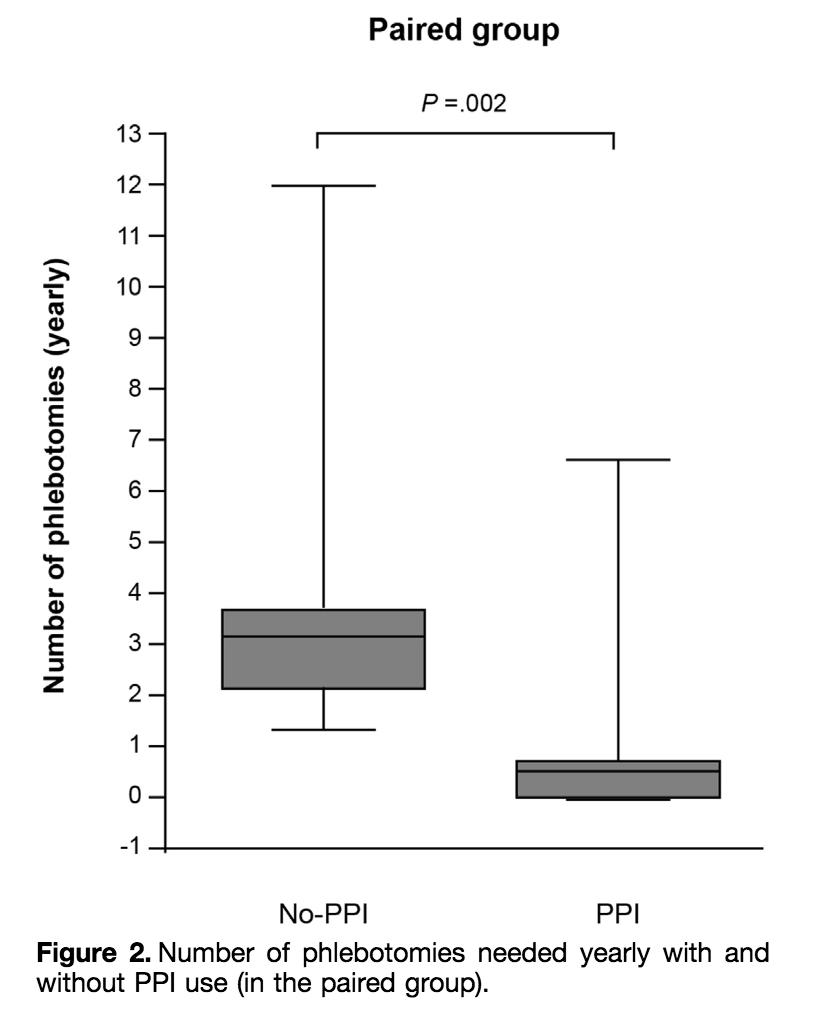
One interesting clinical correlate:
The use of PPIs is associated with a decreased need for phlebotomy in hereditary hemochromatosis!
ncbi.nlm.nih.gov/pubmed/26240005
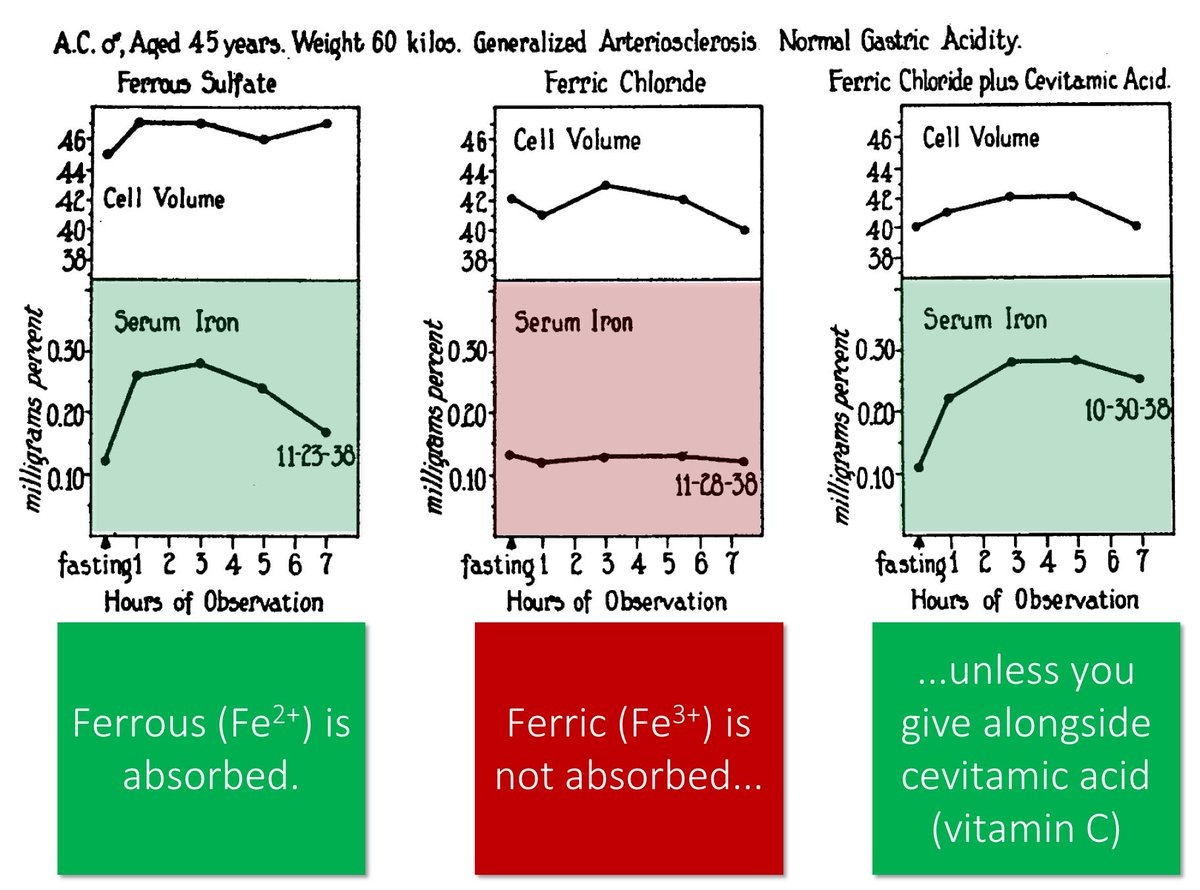
Let's move onto ascorbic acid by going back to the 1939 study.
It also showed that if you give a reducing agent alongside Fe³⁺, it IS absorbed. The reducing agent used in this study: cevitamic acid.
Another name for cevitamic acid: ascorbic acid!
ncbi.nlm.nih.gov/pubmed/16694687
Just like gastric acid, ascorbic acid reduces Fe³⁺ to well-absorbed Fe²⁺.
It isn't the property of being an acid that's key; it’s the ability to act as a reducing agent. Other reducing agents that augment iron absorption: cysteine and fructose!
ncbi.nlm.nih.gov/pubmed/14058578

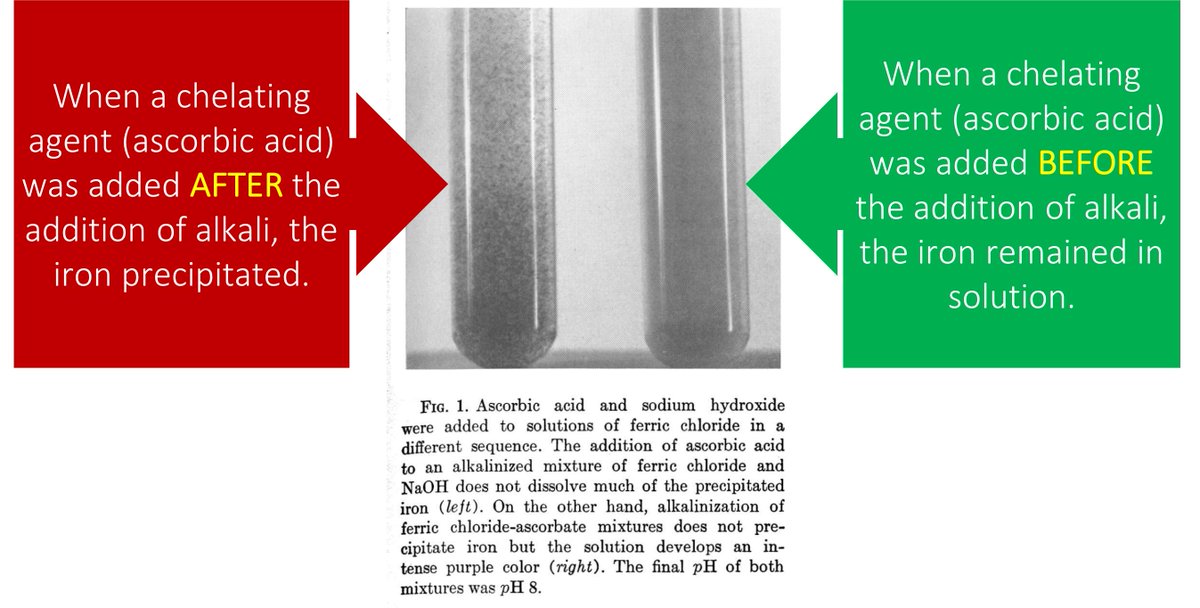
Tweet 7 raises a question: When iron hits the duodenum and the pH rises why doesn’t it precipitate?
The answer: ascorbic acid chelates with iron! These chelates “protect” iron from the rise in pH, keeping it in solution and allowing absorption.
ncbi.nlm.nih.gov/pubmed/5663503
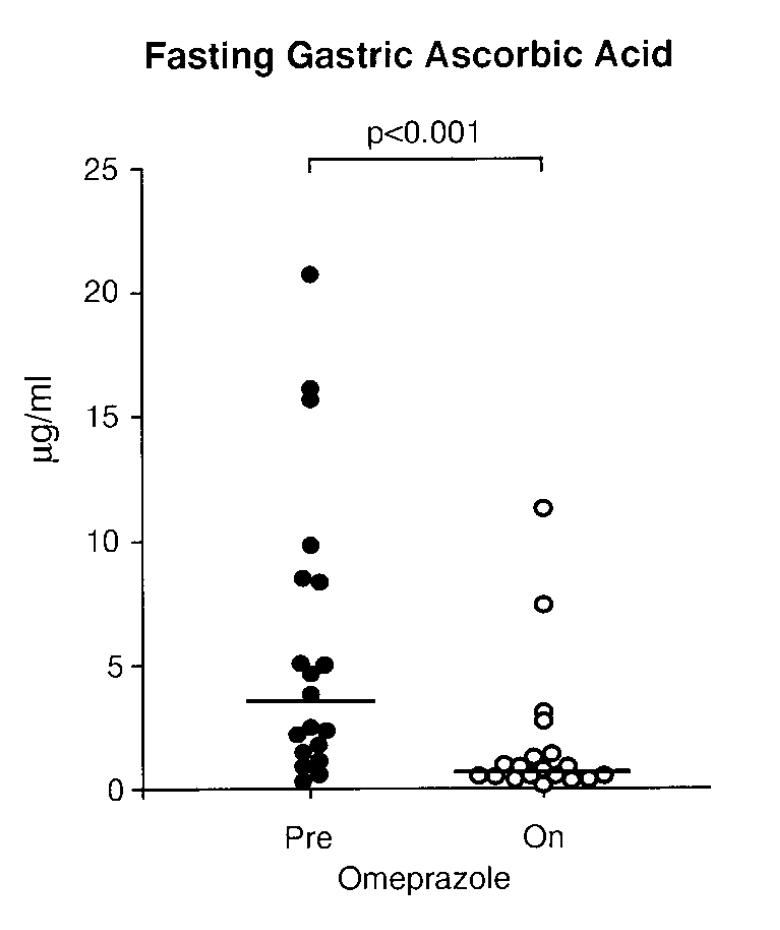
Let’s tie these pieces together with one last observation: ascorbic acid is also secreted into the stomach.
And: PPIs reduce stomach concentrations of hydrochloric acid AND ascorbic acid!
Result: This may amplify the iron deficiency seen with PPIs.
ncbi.nlm.nih.gov/pubmed/10092303
Before summarizing, let's re-ask a version of the original question.
What is it about ascorbic acid that promotes absorption of ingested iron?
✔️non-heme iron is mostly in the poorly absorbed ferric (Fe³⁺) form
✔️HCl promotes absorption via reduction to the well absorbed ferrous (Fe²⁺) form
✔️PPIs decrease the HCl effects on absorption
✔️ascorbic acid promotes absorption by reducing AND chelating iron
Thanks to @CPSolvers and @medrants for inviting me to supplement their discussion.
For more awesome podcast content on iron, I highly recommend:
@COREIMpodcast: clinicalcorrelations.org/2017/11/15/cor…
@thecurbsiders: thecurbsiders.com/internal-medic…
They've got your commute well covered.