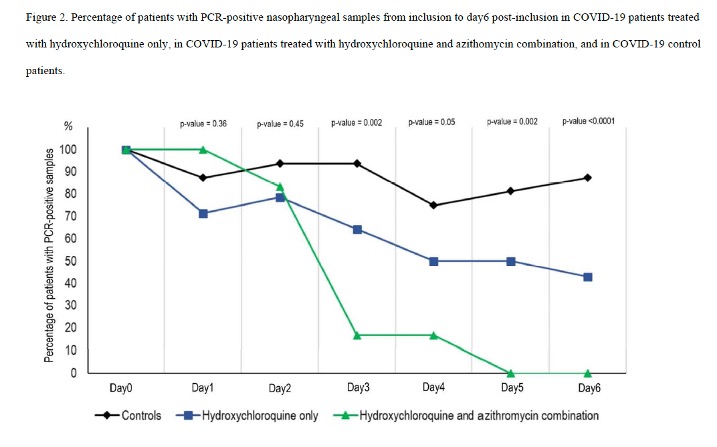
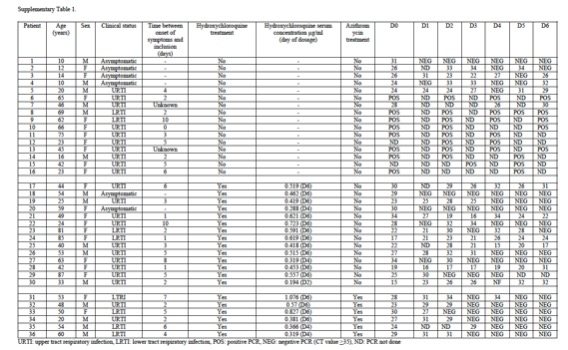
HCQ monotherapy (Ct <23): 1/5 (20%)
HCQ monotherapy (Ct 23+): 7/9 (78%)
HCQ + Azithro (all Ct 24+): 6/6 (100%)
1) This study had a lower threshold for "negative" than most and used as less sensitive swab sample
2) There were a decent number (23%) of total HCQ patients who were not eligible for analysis, but at least five could be considered failures.
2b) These failures would impact monotherapy vs combination therapy (unclear how as they are not described)
3) When correcting for burden of disease/viral load, HCQ and HCQ + AZ look extremely similar