1/As promised & after reading $CRSP latest Q4 2022 #financial #report here is my impression regarding @CRISPRTX latest corporate status. I have focused only on the main issues that I found to be the most interesting & relevant. #CRISPR #BioTech #FinTwit #Genomics #GeneEditing 🧵 

2/IMO the most significant corporate event in Q4 was $CRSP announcement that regulatory Exa-cel submissions of both #SickleCell & #BetaThalassemia validated in the #EU & #UK & that the @US_FDA BLA submission is on track by the end of Q1 ‘23 - possibly reaching the #markets in ‘23 

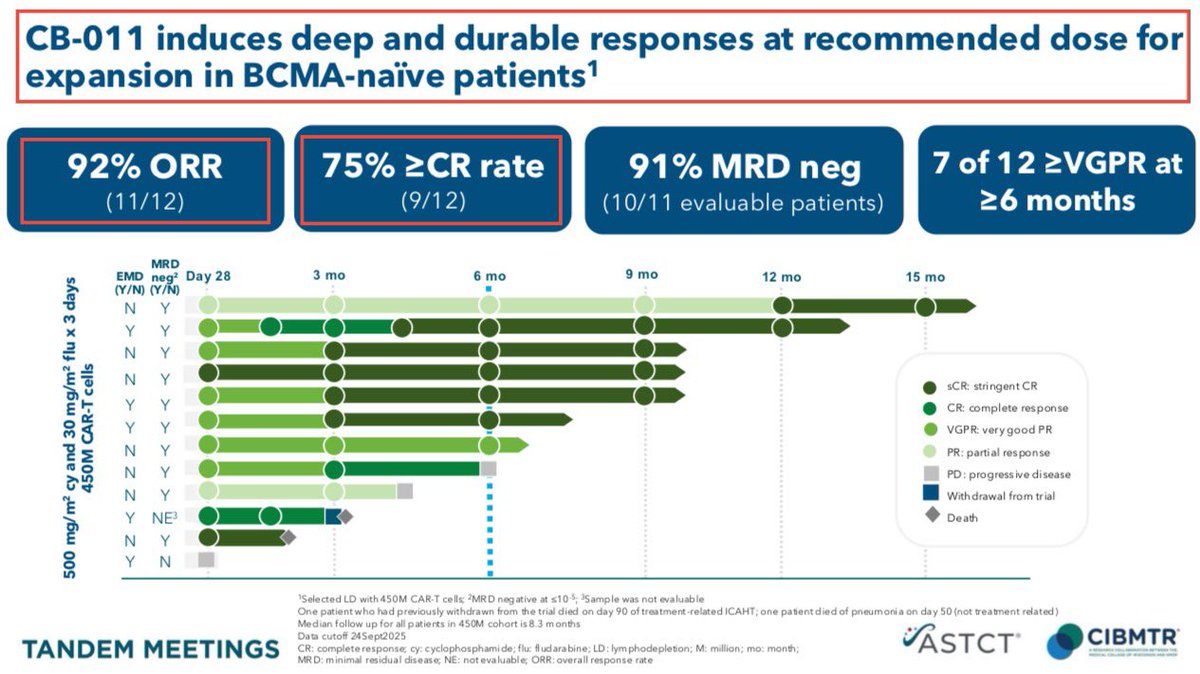
3/Exa-cel/CTX001 - the key program in $CRSP portfolio, is an #autologous Ex-Vivo #CRISPR/#Cas9 #GeneEditing therapy aimed for patients suffering from #TDT or severe #SCD. The latest readout for both programs was phenomenal with 42/44 TDT & 31/31(!) demonstrated remarkable results 

4/Last December - during #ASH22 @CRISPRTX & $VRTX presented an updated clinical data from the exa-cel trail taken from 75 #patients: 44 TDT+31 #sicklecelldisease & with a long follow-up. The data demonstrates that exa-cel has the potential to be a one-time functional #cure.
https://twitter.com/yaireinhorn/status/1602307570609389568
5/Exa-cel was submitted to the @US_FDA for BLA rolling review with expected completion of the submission package by the end of Q1 2023. If approved - Exa-cel will be the first ever #CRISPR product to be commercialised & marketed thus making @CRISPRTX the first to sell a product. 

6/@CRISPRTX second program is CTX110 - a wholly owned donor-derived #GeneEditing #allogeneic antigen receptor T cell #CAR-T #therapy targeting CD19+ B-cell malignancies. CTX110 has also been granted a Regenerative Medicine Advanced Therapy - #RMAT designation from the @US_FDA. 

7/Patients that were treated with CTX110 as part of the @CRISPRTX #CARBON #clinicaltrail have shown good results. CTX110 was well tolerated across all dose levels & patients in CR remain clinically well without receiving any systematic anti-#Cancer #Therapy other than CTX110👇 

8/@CRISPRTX has recently provided a clinical update for both its Part A & Part B of $CRSP ongoing Phase 1 #CARBON trial evaluating the safety and efficacy of CTX110 its wholly-owned #allogeneic CAR T #celltherapy targeting CD19+ B-#cell malignancies. Here is a 🧵 that I wrote👇
https://twitter.com/yaireinhorn/status/1609238227944177668
9/@CRISPRTX will initiate clinical trials for CTX112 - next gen CAR T platforms targeting CD19+ B-cell malignancies, in 1H 2023. CTX112 incorporates the edits in CTX110 plus additional edits to the genes encoding Regnase-1 & TGFBRII, thus increasing the potency of the CAR T cells 

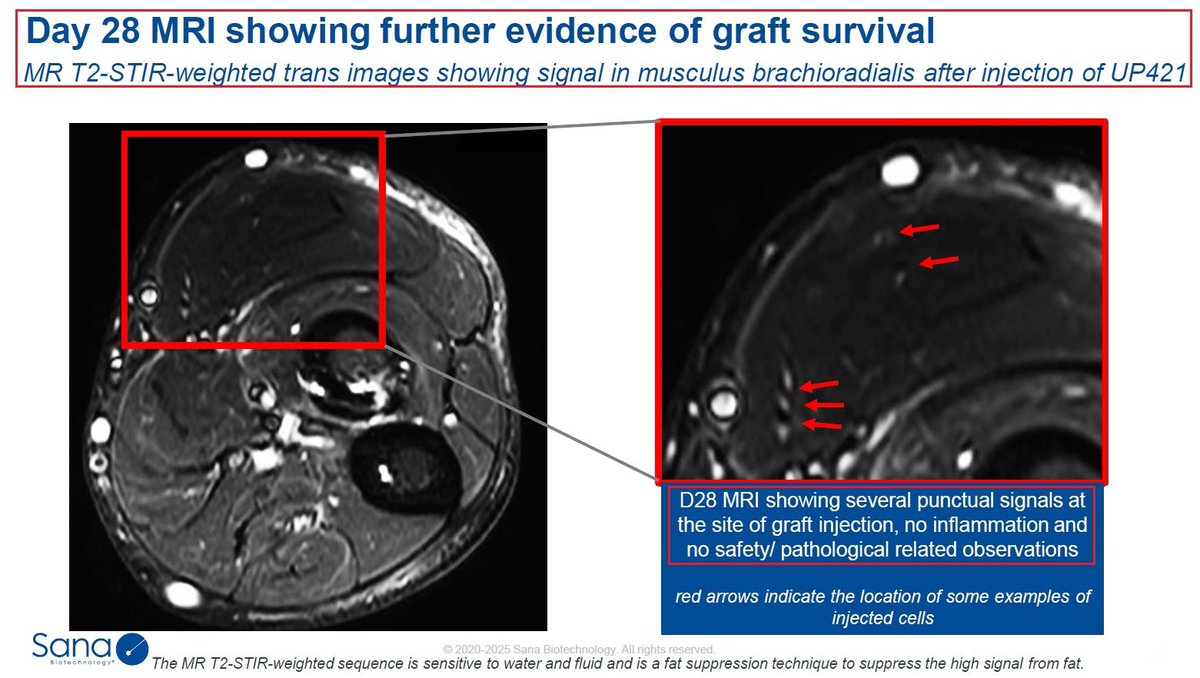
10/CTX130 is another $CRSP wholly-owned #allogeneic CAR-T #CellTherapy targeting #CD70, for the treatment of Mycosis Fungoides & #Sézary Syndrome - both types of cutaneous T-cell #lymphoma. In September @CRISPRTX announced that the @US_FDA has granted it RMAT designation. 
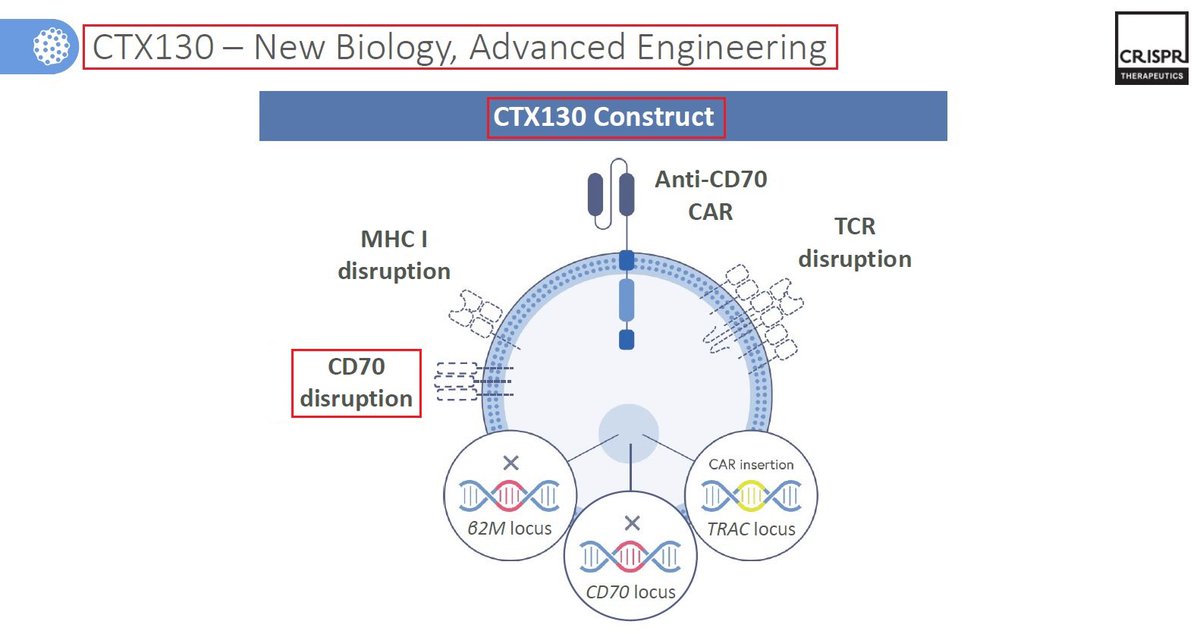
11/CTX130 has showed overall a good safety profile - patients that were treated with CTX130 as part of the @CRISPRTX #COBALT #clinicaltrail have shown good results with #disease control rate DCR of 90% (N=10), 70% ORR & 30% CR rate. $CRSP CTX130 recent readout was also promising
https://twitter.com/yaireinhorn/status/1544355923820519424
12/Two interesting collaboration that @CRISPRTX’s has entered recently are: 1)@MoffittNews for developing new #autologous CAT-T #CellTherapy targeting CD83 for treating #AML. 2)@RoswellPark for developing new autologous CAT-T #therapy targeting GPC3 against solid #tumors. #Cancer 

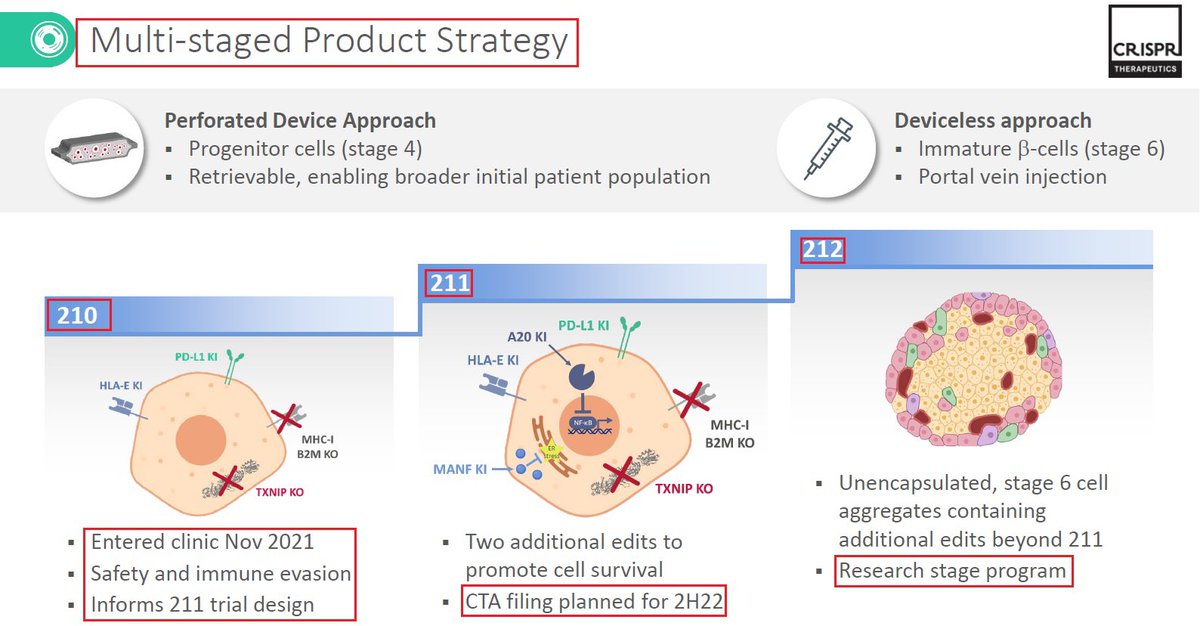
13/@ViaCyte & $CRSP have 3 #CRISPR #GeneEditing programs - VCTX210 in which the first patient was dosed as part of a Phase 1 clinical trial. $CRSP expects to move the other two In-Vivo programs - VCTX211 & VCTX212 - both for #diabetes into the clinic in the next 18-24 months👇 
14/@CRISPRTX has named recently another 2 new #CardioVascular programs 1)CTX310 for #ANGPTL3 & 2)CTX330 for PCSK9. Regarding both - IMO $VERV is much more advanced & especially after @VerveTx’s recent data & the ongoing #Heart-1 trail it is most likely to dominate this market. 

15/As of 12/31/22 $CRSP had capital resources of $2.24B & R&D expenses of $103M. IMO @CRISPRTX’s current cash position will enable it to continue to develop its clinical pipeline with hopefully the first #CRISPR commercial product hitting the markets in 2023 & generating revenue 

16/With an highly anticipated first ever @US_FDA approval for a #GeneEditing platform expected in 2023 @CRISPRTX will be the 1ST #CRISPR company with a product in the market & with a strong cash position of $2.24B - @CRISPRTX IMO continues to look very solid & promising. $CRSP 
And as always please do feel free to #share & #retweet so that those on #Twitter / #FinTwit who are interested in #GeneEditing, #CRISPR, #BioTech & #Genomics will get this relevant data. Wishing you all a great #Week!
• • •
Missing some Tweet in this thread? You can try to
force a refresh