The world of medicine is rapidly dividing into two:
- a new, contemporary medicine, orgs and people who understand data;
- the old, and outdated.
THREAD...
For example, the Health Research Authority is responsible for running ethics committees throughout the country @HRA_Latest The HRA receive substantial and reasonable criticism in the report...

"Having reflected on the evidence submitted to the committee by ourselves and others earlier this year, we have already begun to take action on some of the areas raised in today’s report."...
hra.nhs.uk/about-us/news-…

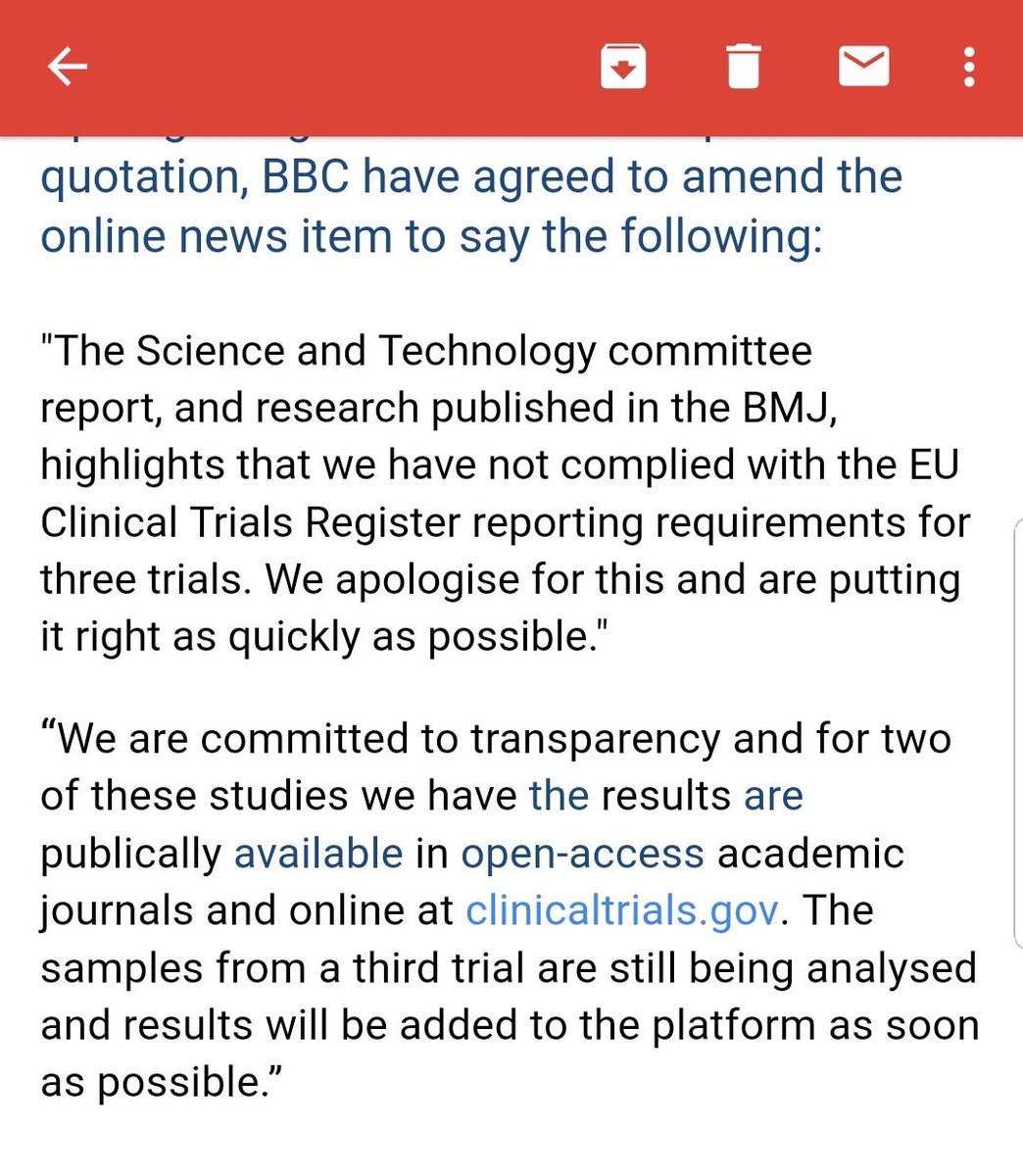
Public Health England have failed to comply with EU rules that require all trial results to be reported directly onto the EU trials register within 12 months of completion...
bmj.com/content/362/bm…
I nearly FELL OVER when I was shown this.
Remember, our site checks reporting compliance for thousands of trials....
eu.trialstracker.net/sponsor/public…

publications.parliament.uk/pa/cm201719/cm…

They told the BBC these trials are already reported:
bbc.co.uk/news/amp/healt…
"We are committed to transparency and have already published this data publicly in academic journals and online at clinicaltrials.gov ."
...
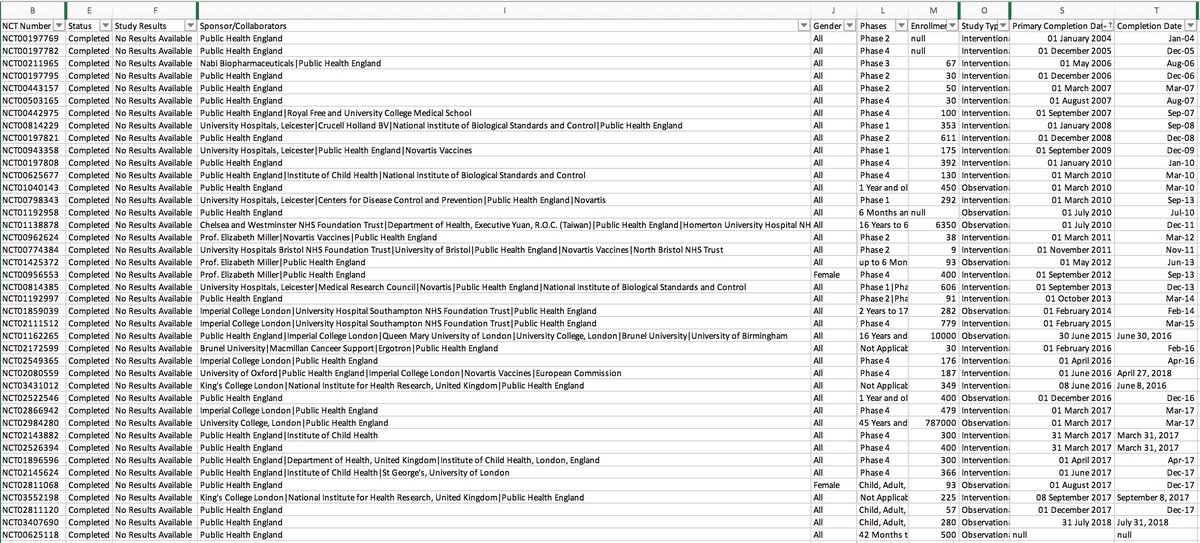
They have three trials breaching EU rules.
You can see them all here.
eu.trialstracker.net/sponsor/public…
One has reported in a journal. One in a conference abstract. One, the largest, is not reported anywhere....
But let's not forget: PHE aside, we now have amazing progress. HRA doing the right thing. EMA doing the right thing. Industry compliance skyrocketing. ONWARD!!
ebmdatalab.net/iqwig-response…
ebmdatalab.net/the-eu-trialst…