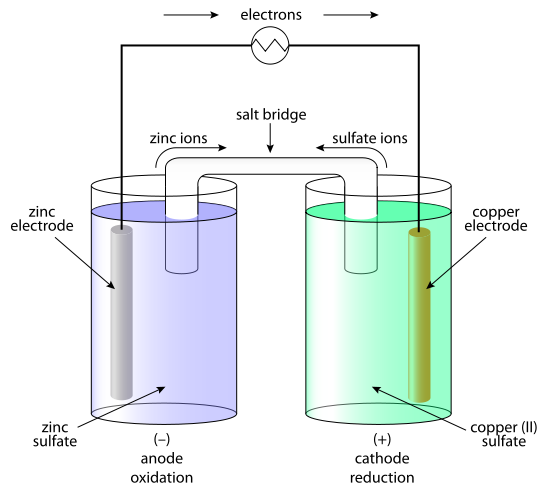
This is a classic 'redox' reaction - Zn transfers 2 electrons per Zn atom to Cu2+.
(img cred: en.wikipedia.org/wiki/Daniell_c…)
First, I need to go and play badminton - because if there's one important part of being a real scientist, it's having hobbies and a good work-life balance 🙂