ec.europa.eu/commission/sit…
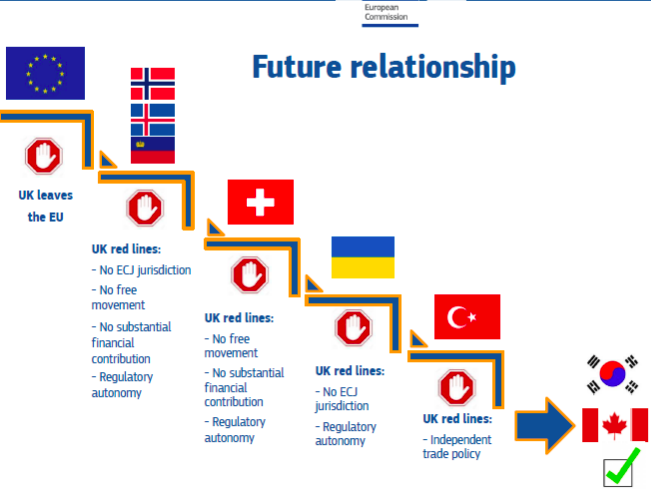
Under these FTAs, Canada and Korea (that is, South Korea) do not generally bind themselves to adopt or conform to a EU laws and regulations.
Take CETA, for
trade.ec.europa.eu/doclib/docs/20…

wto.org/english/docs_e…

The matter is hardly more than extension of the principle that any exporter complies with the product regulations of the countries it is exporting to. If conformity to these regulations is to be assessed by locally established certifiers, then it is hardly
(to be continued)
It is conceivable that the arbitration panel could have to decide on the interpretation of a clause in Art R17 of Decision 768/2008. It is sometimes said that the EU does not allow its law to be
It is unacceptable for a similar provision, if there is one, in a future UK-EU FTA, to be subject to rulings on interpretation by the ECJ. This CETA example shows there is no necessity for ECJ referral in such a case.


Each Party accepts certificates of GMP compliance that have been issued by 'equivalent' regulatory authorities of the other:



























