So, the FDA approves Brexanolone for postpartum depression - a 60-hour continuous infusion at about $25K - with this accompanying picture 😡👇
I refuse to let this pass in silence...
bit.ly/2uhwGKR

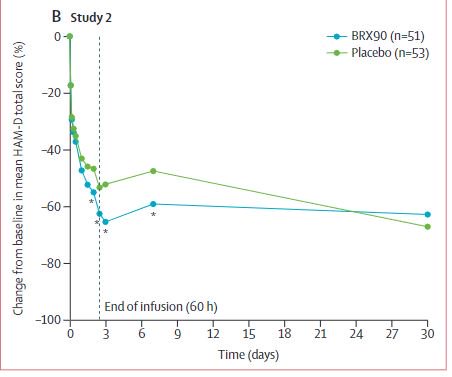
Approval is based on two Pivotal phase III trials reported in Lancet comparing infusions of this drug to placebo in two populations of postpartum women with moderate and high HAM-D scores, respectively: bit.ly/2TPprJA

Effect after 60 hours clinically meaningful effect against placebo for high HAM-D baseline scores. After 7 days not all that impressive.
Y-axis: why not use actual changes in HAM-D instead of %-changes?
After a few weeks?

Adverse reactions: This is just -pun intended- unconscious:
3-5%: Loss of consciousness among those receiving brexanolone?!? Really?!
Severe/serious AE or discontinuation among 10% receiving brexanolone!?


No active comparator. Is this new fancy drug superior to -say- an SSRI?
We do not know and likely never will.
But loss of consciousness does not occur among 3-5% of patients receiving an SSRI.
Post Scriptum:
Generic sertraline in Denmark is $0.05-0.10 daily.
Yes, you read that correctly..🤔



