

But it is different with our best journals. I would rather see a hundred more papers on dog rape at parks in obscure journals than see one more paper in Science that ignores a hundred years of chemical structural understanding.
Science then also published a glowing commentary on the paper. And then C&E News wrote it up, with more glowing comments. And people here tweeted it out.
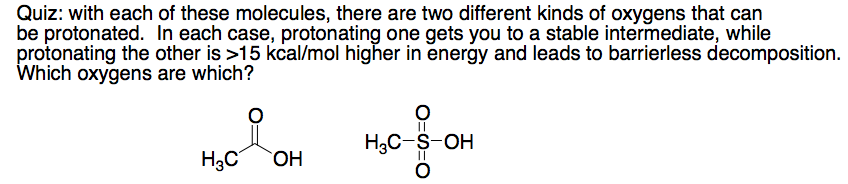
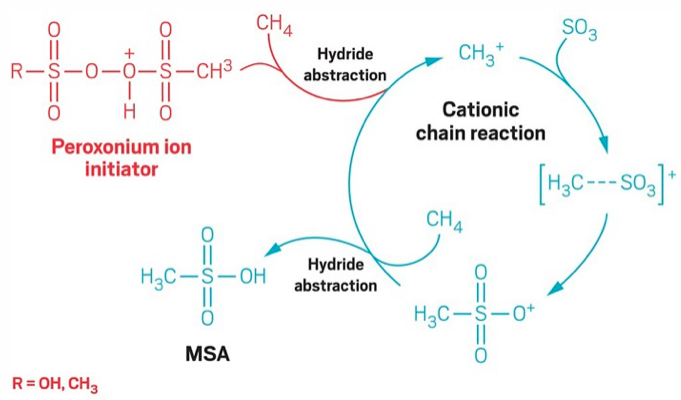
If by some miracle a methyl cation did manage to react with an SO3, reaction at the oxygen give an ok cation that I
And while it is a relatively minor issue, the peroxide catalyst can't have the structure drawn - such structures fall apart by O--S cleavage. Like RCOOH, sulfonic acids protonate on the double bonded O to put charge on S.