This paper challenges the previously accepted model of how #microtubules / #tubulin #estructure changes upon #GTP #hydrolysis.
elifesciences.org/articles/50155

In this work, thank to the use of several #biochemical, #biophysical, and #bioinformatics techniques, showed that the #tubulindimmer expansion does not occur upon hydrolysis.
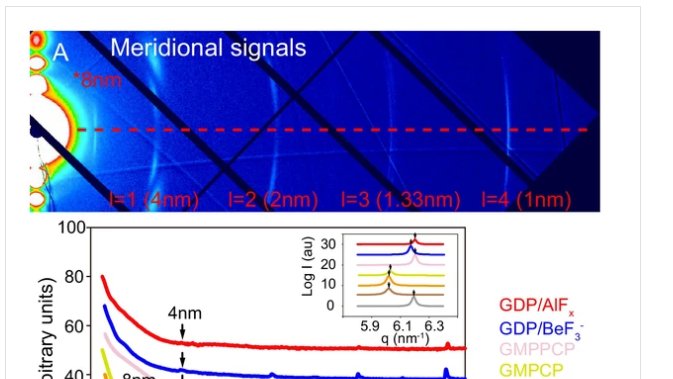
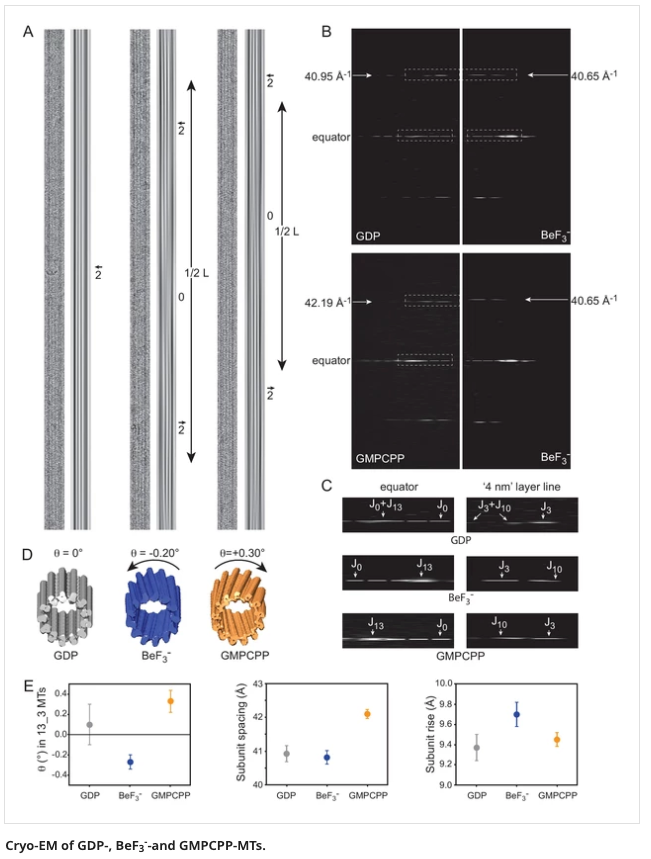

rcsb.org/structure/6GZE
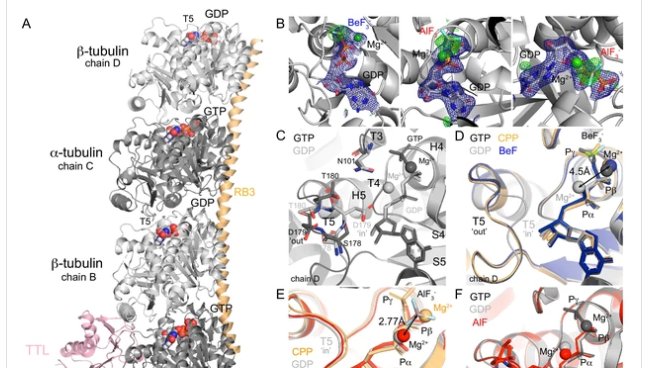
elifesciences.org/articles/50155
Thanks for reading and sharing!