Happy 2021! @MBVanElzakker and I are excited to share our new article published in #Immunometabolism: “Pathogens Hijack Host Cell Metabolism: Intracellular Infection as a Driver of the Warburg Effect in Cancer and Other Chronic Inflammatory Conditions”: ij.hapres.com/htmls/IJ_1341_…
2/ In the paper, we detail molecular mechanisms by which #viral, #bacterial, and #parasite intracellular pathogens can induce, or contribute to, a Warburg-like #metabolism in infected host cells in order to meet their own replication and nutritional needs. 
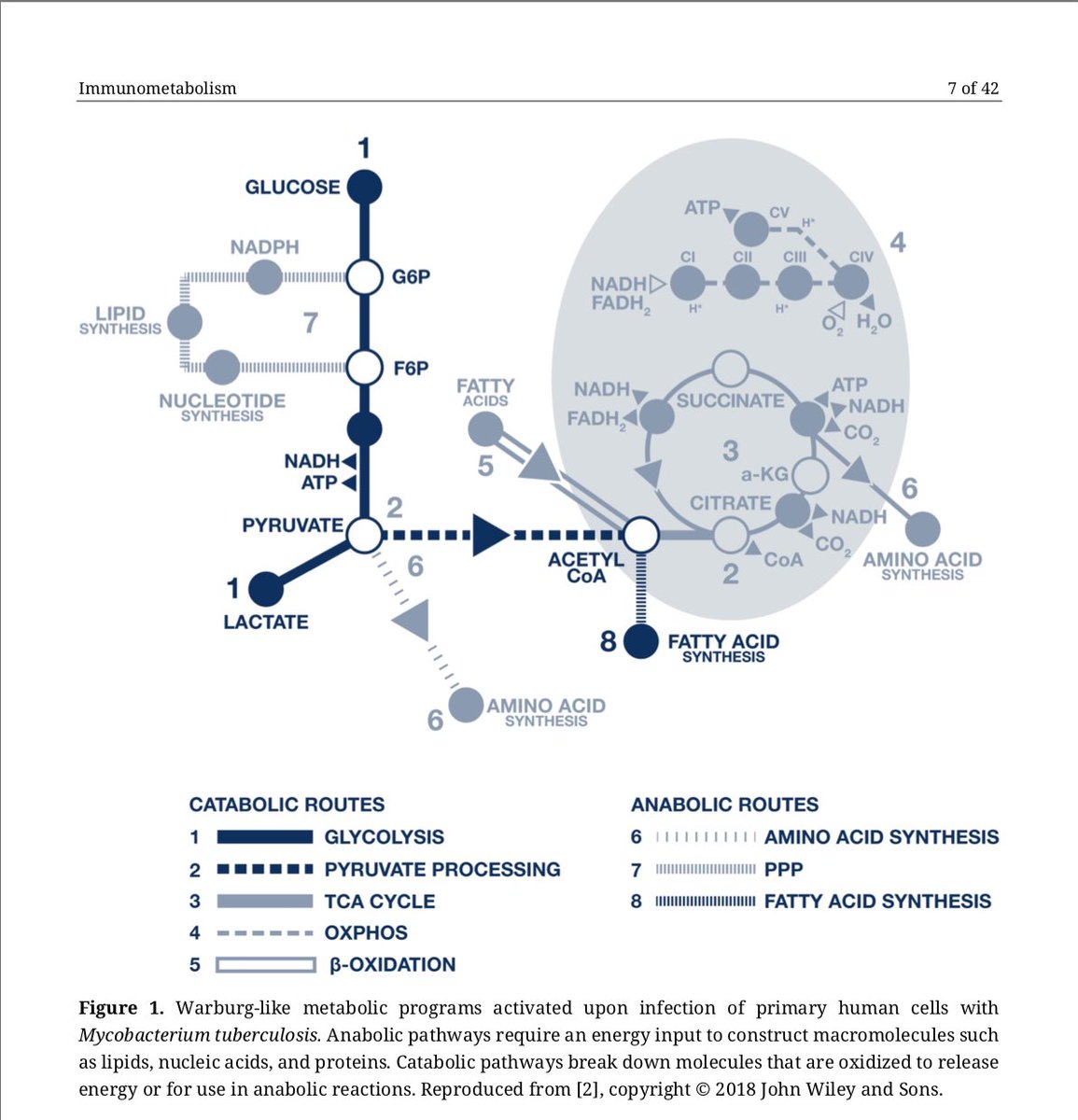
3/ We also discuss how host defense towards #infection may impact cellular metabolic changes (including how #mitochondria can participate in the innate immune response towards infection) 
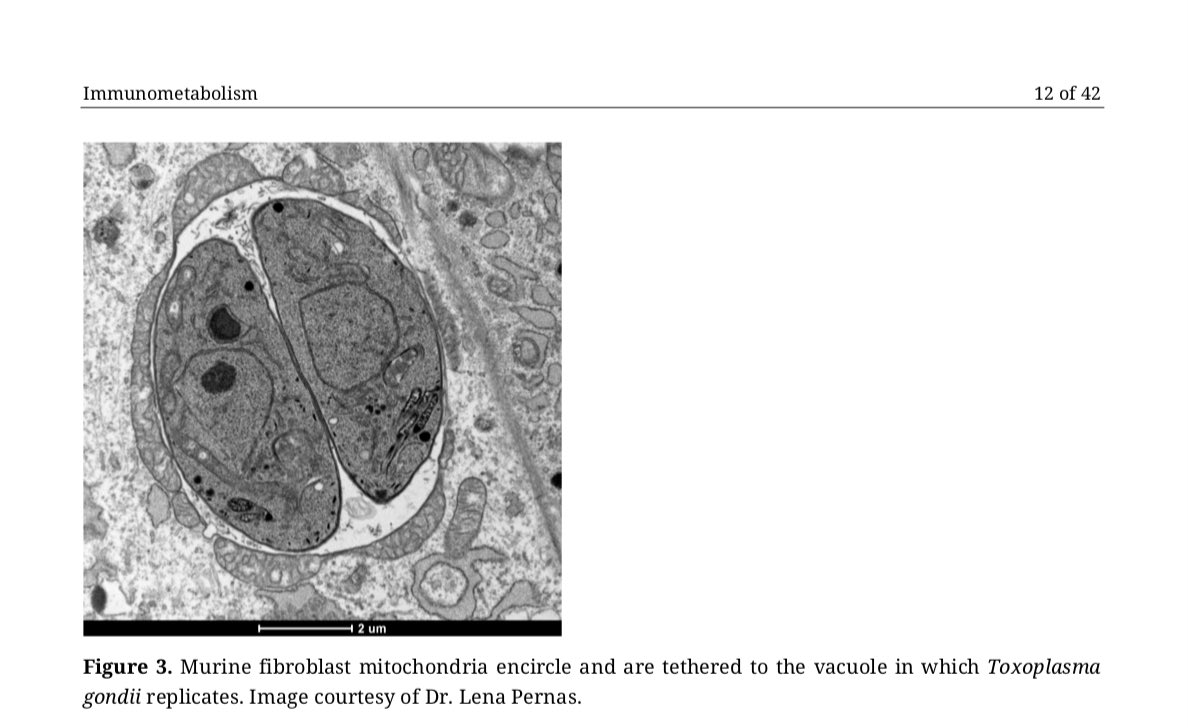
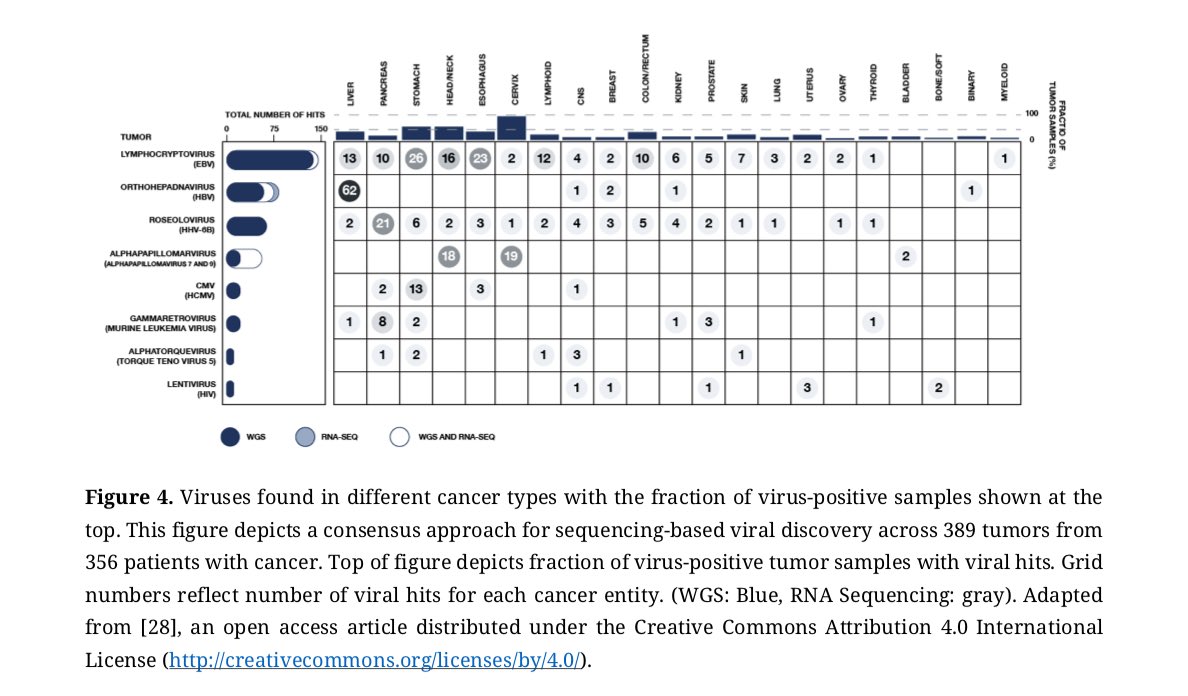
4/ We then provide examples of how many of these same intracellular pathogens have been identified in #tumors, atherosclerotic lesions, #granuloma, and other tissues containing cells with a Warburg or altered metabolism. 
5/ Last, we examine further trends associated with infection and host cell metabolism, including how #pathogen-driven hijacking of host cell lipid metabolism can support viral, bacterial, and parasite survival and replication. 
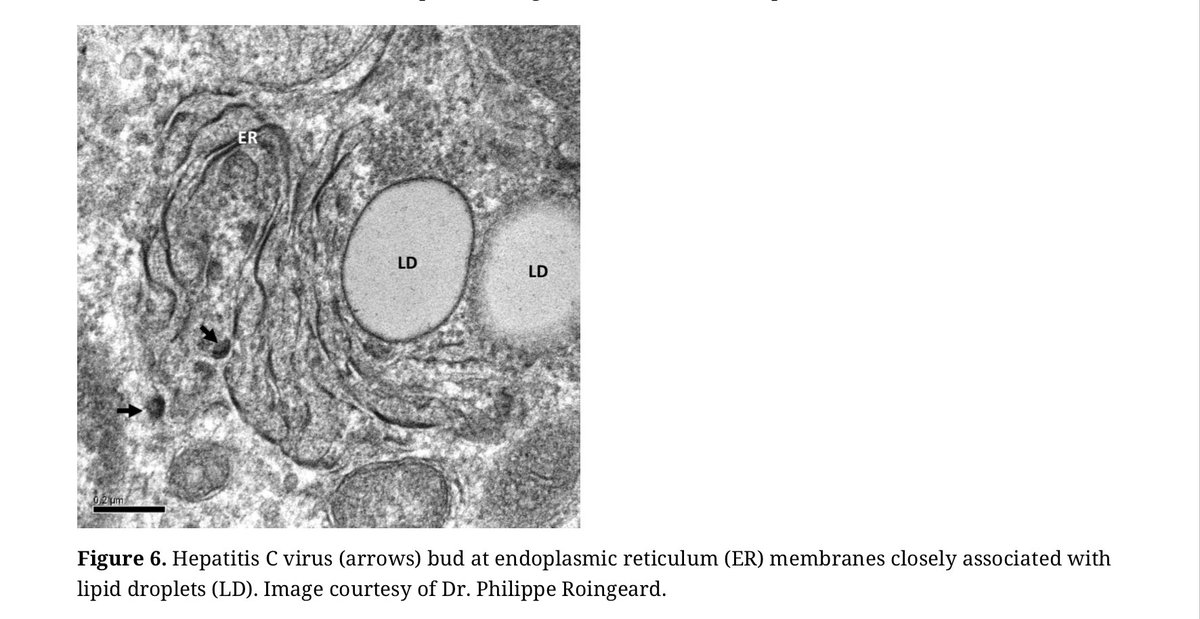
6/ Overall we argue that, at least in a subset of patients w/ #cancer, #atherosclerosis, #sarcoidosis + related conditions, metabolic changes and inflammatory cascades central to the disease process are driven by intracellular pathogens
7/ We also discuss how pathogen-driven hijacking of cellular metabolism may contribute to #MECFS. ME/CFS is an extremely debilitating #neuroinflammatory condition that often begins with an infection (w/ #herpesvirus or enterovirus involvement already documented in some cases) 

8/ A better understanding + acceptance of the trend could result in novel #diagnostics and treatments for patients w/ such conditions (w/ discussion of how a #ketogenic diet might impede pathogen-hijacking of host metabolism)
9/ So many cool people helped make the paper a reality. Special thanks to @lenapernas @Palmer_IMet_lab @dbkell, Dr. Wonder Drake, and @krisfobes for helping us with feedback and on earlier drafts of the manuscript.
• • •
Missing some Tweet in this thread? You can try to
force a refresh